Why Is Zncl2 Colourless
There exist nine different crystalline forms of zinc chloride that are currently known. These hydrates of ZnCl2 are either white in colour or colourless. All of them are highly soluble in water. Zinc chloride exhibits hygroscopic qualities, i.e. it attracts and captures the water molecules in its environment.
Is Ferric Oxide Acidic Or Basic
Ferric oxide, also known as iron oxide, is an amphoteric oxide of iron with the chemical formula Fe2O3. It can be noted that oxides of iron, aluminium, and tin, are all amphoteric chemical species they exhibit both acidic and basic qualities.
Learn more about the chemical properties and importance of Fe2O3 from the expert faculties at BYJUS.
Other important links:
Preview On Related Iron Oxide Crystal Structures
-Fe2O3 crystallizes in a corundum structure characterized by a slightly distorted hexagonal close-packed oxide-ion lattice where 2/3 of the octahedral sites are occupied by Fe3+ ions with non-degenerated energy levels on metal d-electrons due to a ligand field splitting originating from Fe-O hybridization . Oxygen ions that are located parallel to plane levels, are separated by an iron double layer, yielding a stacking sequence of O3-Fe-Fe repeat units. There are three different surface terminations possible for -Fe2O3: a single iron layer , a double iron layer and an oxygen layer .
Figure 1. Schematic representations of various surface terminations for -Fe2O3, Fe3O4, and Fe3O4 surfaces. Red spheres correspond to oxide lattice ions dark blue spheres and cyan spheres represent iron ions on octahedral and tetrahedral lattice sites, respectively.
Overall, due to the great complexity, an unambiguous experimental determination of the surface atomic structure for these iron oxides under different conditions is still a challenging task.
Don’t Miss: Geometry Segment Addition Postulate Worksheet
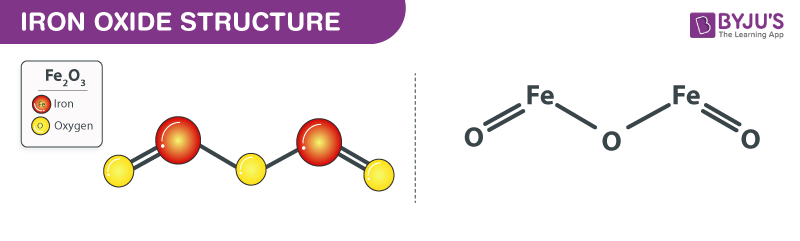
Iron Oxide Structure Fe2o3
The above image describes the structure of the Iron oxide. Fe2O3 is the chemical formula of Iron oxide which has three oxygen atoms, two iron atoms. The oxidation state of Fe2O3 is +3. The bond formation between oxygen and iron depends on the difference in electronegativity between these two atoms. Iron is metal whereas oxygen is non-metal. Therefore, such bonds are called an Ionic bond.
Atoms:
| O |
Why Is This Glaze So Different On These Two Different Porcelains
Why the difference? The one on the right is made from commodity American kaolins, ball clays, feldspars and bentonite. It looks pretty white-firing until you put it beside the Polar Ice on the left . These are extremely low iron content materials. M370 contains low iron compared to a stoneware that iron interacts with this glaze to really bring out the color . Many glazes do not look good on super-white porcelains for this reason.
Recommended Reading: Geometry Segment Addition Postulate Worksheet
Uses Of Iron Oxide Fe2o3
- The ordinary black iron oxide has been used in both copperplate and die stamping inks.
- Oxides of iron constitute the main component of products in the pharmaceutical industry, paint industry, plastic industry, ink industry and cosmetic industry.
- Used as a pigment of natural origin inclusive titanium dioxide.
- Its salt are used as a flocculant in wastewater treatment the dyeing of textiles and the production of fertilizer and feed additives.
- Used as a polishing material in the jewellery trade.
Ceramic Oxide Periodic Table
All common traditional ceramic base glazes are made from only a dozen elements . Materials decompose when glazes melt, sourcing these elements in oxide form. The kiln builds the glaze from these, it does not care what material sources what oxide . Each of these oxides contributes specific properties to the glass. So, you can look at a formula and make a good prediction of the properties of the fired glaze. And know what specific oxide to increase or decrease to move a property in a given direction . And know about how they interact . This is powerful. And it is simpler than looking at glazes as recipes of hundreds of different materials .
Read Also: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Co Adsorption On Fe3o4 And Fe3o4
In order to aid the assignment of the CO bands and to gain a thorough understanding of the structural evolution of the -Fe2O3 surface during reduction, we have carried out additional reference measurements on Fe3O4 and reconstructed Fe3O4-2 ×2R45° single crystal surfaces. Figure 6a shows p-polarized IRRAS data recorded after saturating the slightly and strongly reduced Fe3O4 single-crystal surfaces with CO at 65 K, which is much lower than the Verwey transition temperature of ~120 K . The deconvoluted spectra display four CO stretch bands at 2,166, 2,150, 2,104, and 2,085 cm1 , in excellent agreement with the results found for the highly reduced surface of hematite -Fe2O3 .
Figure 6. Structural evolution of well-defined Fe3O4 single-crystal surfaces after slight and heavy Ar+-sputtering treatment. Afterward such treatment the sample was annealed at 920 K in 106 mbar oxygen. The p-polarized IRRAS data of CO adsorption on reduced Fe3O4 surfaces. The clean surfaces was saturated with a dose of 1 L CO at 65 K. LEED patterns of the corresponding Fe3O4 surfaces recorded at 90 eV.
Table 1. CO stretch frequencies collected on regular and restructured hematite as well as on magnetite and single-crystal surfaces.
Figure 7. Temperature-dependent IRRA spectra starting from CO saturated surfaces of Fe3O4 and Fe3O4 at 65 K and subsequently heating to indicated temperatures. All the spectra were recorded at an incidence angle of 80° with p-polarized light at 65 K.
Co Adsorption On The Pristine
In Figure 2 we display polarization-resolved IRRAS data recorded after a saturating exposure of the pristine stoichiometric -Fe2O3 single crystal surface to CO at 65 K. For p-polarized light, we observe a single, symmetric band at 2,169 cm1 which is characteristic for CO bound to the coordinatively unsaturated surface Fe3+ cations , indicating the presence of a well-defined surface with only one adsorption site. As mentioned above, the surface termination of iron oxides depends strongly on the preparation conditions . For example, the ferryl termination was observed for -Fe2O3 thin films after oxidation with O2 partial pressures of up to 1 mbar, showing a typical IR band at 2,185 cm1 , which is not seen in the present IR spectra . Our IRRAS data provides direct spectroscopic evidence that the pristine -Fe2O3 surface is single Fe3+-terminated.
Figure 2. IRRAS results for CO adsorption on the well-ordered stoichiometric -Fe2O3 surface. Polarization-dependent IRRAS data recorded after exposing the clean surface of -Fe2O3 to 1 L CO at 65 K. The adsorption model is shown as inset. Temperature-dependent IRRAS data starting from a saturated CO coverage at 65 K. Here, all spectra were recorded at an incidence angle of 80° with p-polarized light. The relative CO band-intensity is shown as a function of the sample temperature.
You May Like: Geometry Segment Addition Postulate Worksheet
Let Us Check The Physical Properties Of This Oxide
-
The formula of this oxide is Fe2O3
-
The molecular mass of this oxide is 159.69 g/mole.
-
The density of this oxide is 5.242 g/cm3.
-
The melting point of Iron III Oxide is 1566 °C.
In this section, you will find out how Iron III Oxide is formed by chemically reacting with oxygen and forming the electrovalent bonds. The proper description of the chemical structure along with an illustrated image will help you grab hold of the concept properly. In fact, you will also find how the systematic name for Fe2O3 is given by the Chemistry experts. Understand the importance of the name and how it is assigned to this oxide for future references. By learning the method, you can also understand and discover how other oxides are named.
Difference Between Feo And Fe2o3
November 19, 2020 Posted by Madhu
The key difference between FeO and Fe2O3 is that FeO has iron in +2 oxidations state, whereas Fe2O3 has iron in +3 oxidation state.
In brief, FeO and Fe2O3 are oxides of iron but having the iron atoms in different oxidation states. FeO is the chemical formula of iron oxide while Fe2O3 is the chemical formula of iron oxide. These two substances have different chemical properties, appearances, as well as different uses. In this article, we will be discussing these properties and comparing them to discern the difference between FeO and Fe2O3.
You May Like: Which Founding Contributors To Psychology Helped Develop Behaviorism
What Is The Difference Between Feo And Fe2o3
FeO and Fe2O3 are oxides of iron having different oxidation states of iron atoms. The key difference between FeO and Fe2O3 is that FeO has iron in +2 oxidations state, whereas Fe2O3 has iron in +3 oxidation state. Moreover, FeO is a black powder while Fe2O3 is a red powder.
The following infographic tabulates more differences between FeO and Fe2O3.
What Is Black Iron Oxide Used For
Black iron oxide or magnetite is used for resistance to corrosion, too. Often used in anti-corrosion paints is black iron oxide . Iron oxides are used to shorten proton relaxation times as a contrast agent in magnetic resonance imaging.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Also Check: What Is The Molecular Geometry Of Ccl4
What Is Iron Oxide Made Of
Iron oxides are compounds that are composed of iron and oxygen. There are seventeen known iron oxides and oxyhydroxides, of which the best known is rust, a type of iron oxide. Iron oxides and oxyhydroxides are common in nature and play a significant role in many processes, both geological and biological.
Iron Iii Oxide: What Are Its Formula And Chemical Properties
Iron III Oxide is one of the most common oxides you have studied in the previous classes. You will find more elaborate information related to this oxide when you advance to a higher class. This time, a section of the chapter has been dedicated to this oxide so that you can learn how it behaves in different physical and chemical conditions.
The chemical formula of Iron III Oxide is Fe2O3. It is obtained from a mineral ore called hematite. It is mined and then processed to obtain iron oxide for extracting the metal. Iron is also called Ferrum in Latin. The valency of this metal is 2 and 3. When it combines with valency 3, it is called Ferric. When it combines with other elements showing valency 2, it is called Ferrous. In this segment, we will study about this Iron Oxide and its chemical properties. The IUPAC name of Fe2O3 is Ferric Oxide. This oxide occurs in nature in a neutral state as it is properly balanced in terms of valence electrons.
You May Like: Segment Addition Postulate Worksheet Answer Key
What Is Fe2o3 Xh2o
Iron oxide is also referred to as rust with the formula Fe2O3 xH2O, and this label is useful to some degree, since rust has many properties and a similar structure, but rust is considered an ill-defined substance in chemistry, described as hydrous ferric oxide.
The compound formed when iron reacts in the air with oxygen is ferric oxide. It has a reddish-brown hue and is also known as the hematite and rust mineral. Ferrous oxide is black and is referred to as the mineral wustite.
Was this answer helpful?
Production Of Iron Oxide:
Iron oxide is a product obtained from the oxidation of iron. In laboratories, it is prepared by electrolyzing sodium bicarbonate solution, an inert electrolyte, along with an iron anode.
The deriving hydrated iron oxide, which is written here as FeOH, dehydrates at around 200 °C. The reaction is as follows:
Read Also: Electron Dot Diagram For Ccl4
Chemical Properties Of Iron Iii Oxide
As you all know that this is an oxide included in the chemistry chapter related to metals, you will have to study its chemical properties elaborately. On this concept page, you will find out how the experts have perfectly segmented the properties along with good examples. This elaboration has been done using simple language so that students of all merit levels can understand it properly.
What Is The Systematic Name Of Fe2o3 S
4.7/5Fe2O3Fe2O3
Also asked, what does Fe2O3 mean?
Writing the name for Fe2O3 is a bit more challenging since Fe is a transition metal. This means you need to use parenthesis and Roman Numerals to show the charge on the Fe atoms. In this case each Fe atom has a charge of +3 so we write Fe2O3 as Iron Oxide.
Additionally, is Fe2O3 ionic or covalent? Iron has an electronegativity of 1.83 and oxygen 3.44. The difference is 3.44 – 1.83 = 1.61. The bond character is polar covalent although the difference is very close to that of ionic bonds. So iron oxide is a polar covalent compound with ionic character.
In respect to this, how is Fe2O3 formed?
Iron oxide is a product of the oxidation of iron. It can be prepared in the laboratory by electrolyzing a solution of sodium bicarbonate, an inert electrolyte, with an iron anode: 4 Fe + 3 O2 + 2 H2O 4 FeO The resulting hydrated iron oxide, written here as FeO, dehydrates around 200 °C.
What is the name of FeO2?
Iron oxide
Also Check: What Is Figure Ground Perception Psychology
Uses Of Iron Iii Oxide Fe2o3
After learning the Fe2O3 chemical name and chemical properties, you will proceed to the section where you will find its uses. It is used to manufacture die inks for stamping. It is the prime constituent used in various industries manufacturing pain, plastic, pharmaceutical products, ink and cosmetics. It is also used as a natural pigment.
1. What is the formula for iron 3 oxide?
The formula name of iron 3 oxide is Ferric Oxide. Ferric is used when this metal uses its valency 3. Its formula is Fe2O3. You will learn about this oxides physical and chemical properties elaborately from this concept page.
2. What is Fe2O3?
Fe2O3is a metal oxide formed from the oxidation of iron. It occurs naturally in the ores such as hematite. It acts as a weak alkali when dissolved in water. It also responds in acid-base reaction to form respective salts with different acids.
3. How Iron III Oxide is produced?
You will find the exact process for the production of iron III oxide in the extraction of the metals section of this concept page.
More Information Onmolar Mass And Molecular Weight
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Finding molar mass starts with units of grams per mole . When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom in the formula by the formula weight and multiplying by 100.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass , which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Read Also: Kendall Hunt Geometry Answer Key
Is Fe2o3 Covalent Solid
The difference is 3.44 1.83 = 1.61. The bond character is polar covalent although the difference is very close to that of ionic bonds. So iron oxide is a polar covalent compound with ionic character. Fe2O3 is an inorganic compound containing a central oxygen atom with two iron atoms attached by single ionic bonds.
Main Difference Fe2o3 Vs Fe3o4
Fe2O3 and Fe3O4 are two common oxides of iron that can be found naturally along with some impurities. Fe2O3 is also known as hematite, a mineral from which pure Fe2O3 can be obtained via processing and Fe3O4 is known as magnetite for the same reason. These minerals are the feedstock for pure metal iron production. There are many physical and structural difference between Fe2O3 and Fe3O4. The main difference between Fe2O3 and Fe3O4 is that Fe2O3 is a paramagnetic mineral having only Fe2+ oxidation state whereas Fe3O4 is a ferromagnetic material having both Fe2+ and Fe3+oxidation states.
Read Also: Algebra Age Problems
Let Us Take A Quick Look Into The Chemical Properties Of Iron Iii Oxide Fe2o3
-
Iron III oxide is basic in nature. It forms a weak base when dissolved in water. The name of that base is Ferric Hydroxide 3).
-
Its alkaline properties can be observed when it reacts with an acid to form the respective inorganic salt and water at the end of an acid-base reaction.
-
The oxidation state of the metal in this oxide is +3.
-
It is not soluble in water but strongly soluble in strong acid.
You will find the proper elaboration of these properties along with the iron 3 oxide balanced equation for each one of the points.
Conventional Experimental Techniques X
XRD analysis of the initial -Fe2O3 sample was recorded on a PANalytical X´Pert PRO diffractometer in the Bragg-Brentano geometry, equipped with an iron-filtered CoK radiation source, an X´Celerator detector, a programmable divergence and diffracted beam anti-scatter slits. Generally, 200L of a sample suspension was dropped onto a zerobackground singlecrystal Si slide, allowed to dry under vacuum at room temperature and scanned in continuous mode under ambient conditions. The commercially available standards SRM640 and SRM660 supplied by the National Institute of Standards and Technology were used to evaluate line positions and instrumental line broadening, respectively. The acquired pattern was processed using the X´Pert HighScore Plus software package in combination with the PDF-4+ and ICSD databases.
The room-temperature transmission 57Fe Mössbauer spectrum of the initial -Fe2O3 sample was recorded with a Mössbauer spectrometer operating in constant acceleration mode and equipped with a 50 mCi 57Co source of -rays. The collected Mössbauer spectrum was fitted using Lorentzian line shapes with the MossWinn software package based on the least-square method. The isomer shift values were referenced to a metallic -Fe sample at room temperature.
Recommended Reading: What Does Abiotic Mean In Biology