Accuracy Is Close To A Known Value; Precision Measures Repeatability
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Accuracy and precision are two important factors to consider when taking data measurements. Both accuracy and precision reflect how close a measurement is to an actual value, but accuracy reflects how close a measurement is to a known or accepted value, while precision reflects how reproducible measurements are, even if they are far from the accepted value.
Accuracy And Precision Chemistry Worksheet Answers
Accuracy and precision chemistry worksheet answers a question for the student. It is an easy to follow sheet which will give you step by step instructions on how to do something using the lab bench. Here are some of the benefits of this worksheet.
First, it is very important to have a worksheet that provides for accuracy. This is very important if you are going to do experiments or make calculations. When you get into the lab, you will need to do lots of calculations. If you are not able to do it correctly, you may not get the results that you want. Having an accurate worksheet will save you time and possibly even get you into trouble.
Accuracy and Precision Worksheet from accuracy and precision chemistry worksheet answers , source:fronteirastral.com
Second, an accuracy and precision chemistry worksheet answers a question for the student. You may have a question about what happens when you mix a solution. This may be something that you have not learned in your chemistry class. By having an answer to this question you will know exactly how to react to the solution.
Third, having the correct answer will give you the confidence to do more experiments and calculate more complex reactions. Having the wrong answer could cost you money. Having the correct answer will also give you more confidence because you can rely on knowing that you do not have an error on your work.
What Is The Difference Between Accuracy And Precision Measurements
The terms Accuracy and Precision are critical within science and their meanings are constantly being used incorrectly or misunderstood to mean the same thing.
At Precisa, our work is centred on the production of Precision measurements. This article aims to clear up the confusion, exploring the difference between precision and accuracy and the correct way to use each term, using the example of a dart board to demonstrate.
Read Also: Fsa Algebra 1 Eoc Review Functions And Modeling Answer Key
How To Find The Precision Of Numbers
1) 22.52, 22.48, 22.54 2) 22.64, 22.58, 22.62
which one is the more precise if the target number is 22.52. Like i can figure out which one is the most accurate one but like i really cant understand how to find the most precise one. So thats where i need help. Can you help me to find the precision.
PS: next to the exercise it said “to find the precision calculate the average value for each data set, then calculate the average value of the absolute deviations of each measurement from the average.” – It is telling me how to find the precsision but like i cant really seem to understand the wording… soo it wouldv been great if you could just break it down for me…
– Thanks in advance guys!
ok accuracy is how close to the true value your average is.
precision is a measure of the uncertainty in your measurements.
you can think of precision , in this case, as how careful you were in taking readings and did you make the observation the same way every time.
for instance, if you were measuring 3 m with a meter stick and you took the time to carefully lay it out you might measure, 2.981, 2.983, 2.982; pretty precise.
if you were not careful and just flung the meter stick down you could read 2.950 once and 3.100 once and so on, not very precise
for precison you want to look at how much each measurement deviates from the average.
to take an absolute deviation you first average the number.
the subtract this average from EACH data point you have.
the difference in the deviation.
Error Systematic Random And Gross
Error refers to a lack of accuracy, precision, or both.; Systematic and gross error are controllable, random error is not.; Knowing the type of error can lead to a solution.
Systematic error arises from the experimental design and affects the result in one direction, up or down.;
Gross error arises from an undetected mistake that causes the measurement to be greatly different than the average.; This measurement is called an outlier.; If it is detected it is called a mistake or accident and the experiment is repeated.
Random error arises from nature and affects the result in two directions up and down.
Let’s look at what these look like in your data sets.
Random Error
These results show the scattering of the data above and below the line.; Since the data is “all over the place” or above and below the line it is classified as random.; Scientists have no way to fix random error, so we tell it like it is and report it with standard deviations and R2 values, which come from standard deviations.; Precision is affected, but accuracy is preserved.
Systematic error
This graph shows systematic error in the blue line.; It is consistently above the red line, indicating that something is wrong.; When an experiment generates a result that is greatly above or below a measurement an examination for systematic error is called for.; Accuracy is damaged, precision is not.
Gross error
So there’s three types of error that can happen.
Systematic – all a little up or a little down
Also Check: Exponential Growth And Decay Common Core Algebra 1 Homework Answer Key
Precision Of Measuring Tools And Significant Figures
An important factor in the accuracy and precision of measurements involves the precision of the measuring tool. In general, a precise measuring tool is one that can measure values in very small increments. For example, a standard ruler can measure length to the nearest millimeter, while a caliper can measure length to the nearest 0.01 millimeter. The caliper is a more precise measuring tool because it can measure extremely small differences in length. The more precise the measuring tool, the more precise and accurate the measurements can be.
How To Control Accuracy And Precision
Both accuracy and precision are the goal of any measurement. Variations in accuracy and precision are largely controllable.
In target shooting, you improve accuracy by moving closer to the target, or using an aiming aid like a scope or laser pointer. You improve precision by mounting your gun to a table or bench or shooting indoors out of the wind.
In a lab, improve accuracy and precision by using the same procedure taking each measurement and using tools designed for measurements. For example, you get a more accurate and precise volume measurement using a graduated cylinder than you do using a water glass.
In general, use good tools designed for the measurement you are trying to make and pay close attention to what you are doing when you use them. This helps any measurement you make.
Don’t Miss: What Is The Molecular Geometry Of Ccl4
Summary Accuracy Vs Precision In Chemistry
Accuracy and precision are independent of each other. The difference between accuracy and precision in chemistry is that accuracy reflects how close a measurement to an accepted value whereas precision reflects how reproducible the measurements are.
Reference:
1. Helmenstine, Anne Marie. What Is Accuracy in Science? ThoughtCo. Available here;2. Helmenstine, Anne Marie. Understand the Difference Between Accuracy and Precision. ThoughtCo. Available here;
Image Courtesy:
1.Precision versus accuracyBy CK-12 Foundation ; User:Adrignola File:High School Chemistry.pdf, page 107, via Commons Wikimedia;
Difference Between Accuracy And Precision
In the previous few sections having discussed what each term means, let us now look at their differences.
| Accuracy; | Precision |
| Accuracy refers to the level of agreement between the actual measurement and the absolute measurement. | Precision implies the level of variation that lies in the values of several measurements of the same factor. |
| Represents how closely the results agree with the standard value. | Represents how closely results agree with one another. |
| Single-factor or measurement. | Multiple measurements or factors are needed. |
| It is possible for a measurement to be accurate on occasion as a fluke. For a measurement to be consistently accurate, it should also be precise. | Results can be precise without being accurate. Alternatively, the results can be precise and accurate. |
Also Check: Math Caching Algebra 1 Answers
How Do Accuracy Precision And Error Relate To Each Other
The random error will be smaller with a more accurate instrument and with more repeatability or reproducibility . Consider a common laboratory experiment in which you must determine the percentage of acid in a sample of vinegar by observing the volume of sodium hydroxide solution required to neutralize a given volume of the vinegar. You carry out the experiment and obtain a value. Just to be on the safe side, you repeat the procedure on another identical sample from the same bottle of vinegar. If you have actually done this in the laboratory, you will know it is highly unlikely that the second trial will yield the same result as the first. In fact, if you run a number of replicate trials, you will probably obtain scattered results.
As stated above, the more measurements that are taken, the closer we can get to knowing a quantitys true value. With multiple measurements , we can judge the precision of the results, and then apply simple statistics to estimate how close the mean value would be to the true value if there was no systematic error in the system. The mean deviates from the true value less as the number of measurements increases.
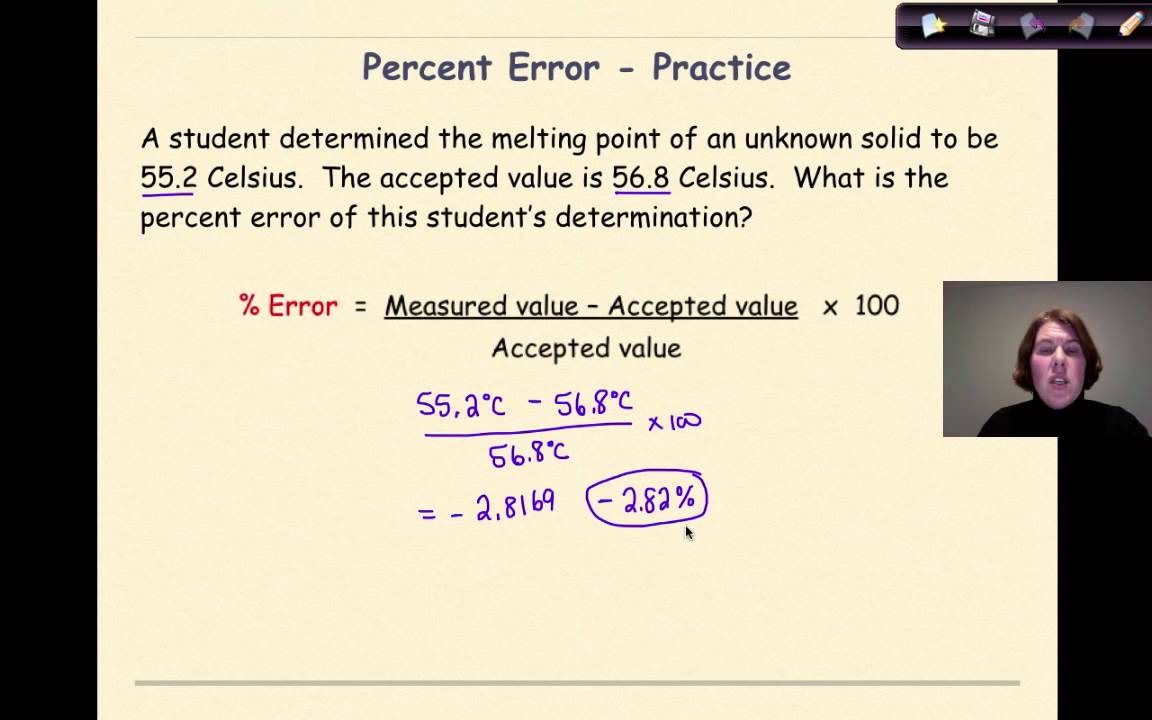
Error and Percent Error YouTube
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
Boundless.
Difference Between Accuracy And Precision In Chemistry
June 25, 2018 Posted by Madhu
The key difference between accuracy and precision in chemistry is that accuracy reflects how close a measurement to an accepted value whereas precision reflects how reproducible the measurements are.
Both the terms accuracy and precision gives the idea of how close a measurement is close to an actual value. But they are different from each other in definition and application. In chemistry, we use both these terms as analytical indicators for the values we get for certain experiments.
Read Also: What Does Abiotic Mean In Biology
Accuracy Vs Precision: Whats The Difference
I remember sitting in my ninth-grade chemistry class when my teacher mentioned that the days lesson would include a discussion about accuracy and precision, and how both relate to making experimental measurements. Ive always been more of a liberal-arts-minded individual, and I initially thought, Is there really a difference between the two terms? In fact, I even remembered using the words interchangeably in my writing for English class!
However, as I continued through more advanced science and math courses in college, and eventually joined Minitab Inc., I became tuned in to the important differences between accuracy and precisionand especially how they relate to quality improvement projects!
Ngss Science And Engineering Practices:
AccuracyPrecision
- If the darts are neither close to the bulls-eye, nor close to each other, there is neither accuracy, nor precision . ;
- If all of the darts land very close together, but far from the bulls-eye, there is precision, but not accuracy . ;;
- If the darts are all about an equal distance from and spaced equally around the bulls-eye there is mathematical accuracy because the average of the darts is in the bulls-eye. This represents data that is accurate, but not precise . However, if you were actually playing darts this would not count as a bulls-eye!
- If the darts land close to the bulls-eye and close together, there is both accuracy and precision .;
Don’t Miss: Holt Geometry Lesson 4.5 Practice B Answers
How Does Significant Figures Relate To Accuracy
Accuracy refers to how exactly the calculated value matches the right value. Precision refers to how closely individual measurements are in accordance with each other. The number of significant figures is the number of digits considered to be accurate by the person doing the calculation.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
What Is Accuracy In Chemistry
Accuracy in chemistry refers to how close a measurement to the true value. In order to increase the accuracy, we have to calibrate the instrument that we use to take the measurement. When calibrating, we should use a proper standard as the reference.
Figure 01: Precision vs. accuracy. is neither precise nor accurate. is precise and accurate. is precise but inaccurate.
The accurate measurement should not have a systemic error or a random error. However, there are always errors occur when we take measurements from an analytical instrument, it can be either instrumental errors or human errors.
Also Check: Geometry Segment Addition Postulate Worksheet
Example : Calculating Percent Uncertainty: A Bag Of Apples
A grocery store sells a 5-pound;bags of apples. You purchase four bags over the course of a month and weigh the apples each time. You obtain the following measurements:
- Week 1 weight: 4.8 lb
- Week 2 weight: 5.3 lb
- Week 3 weight: 4.9 lb
- Week 4 weight: 5.4 lb
You determine that the weight of the 5-pound bag has an uncertainty of ±0.4 lb. What is the percent uncertainty of the bags weight?
Strategy
First, observe that the expected value of the bags weight, A, is 5 lb. The uncertainty in this value, A, is 0.4 lb. We can use the following equation to determine the percent uncertainty of the weight:
Solution
Plug the known values into the equation:
Discussion
We can conclude that the weight of the apple bag is 5 lb;±;8%. Consider how this percent uncertainty would change if the bag of apples were half as heavy, but the uncertainty in the weight remained the same. Hint for future calculations: when calculating percent uncertainty, always remember that you must multiply the fraction by 100%. If you do not do this, you will have a decimal quantity, not a percent value.
Accuracy And Precision Examples
A good analogy for understanding accuracy and precision is to imagine a football player shooting at the goal. If the player shoots into the goal, he is said to be accurate. A football player who keeps striking the same goalpost is precise but not accurate. Therefore, a football player can be accurate without being precise if he hits the ball all over the place but still scores. A precise player will hit the ball to the same spot repeatedly, irrespective of whether he scores or not. A precise and accurate football player will not only aim at a single spot but also score the goal.
The top left image shows the target hit at high precision and accuracy. The top right image shows the target hit at a high accuracy but low precision. The bottom left image shows the target hit at a high precision but low accuracy. The bottom right image shows the target hit at low accuracy and low precision.
Recommended Reading: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
What Is The Difference Between Accuracy And Precision
Both accuracy and precision reflect how close a measurement is to an actual value, but they are not the same. Accuracy reflects how close a measurement is to a known or accepted value, while precision reflects how reproducible measurements are, even if they are far from the accepted value. Measurements that are both precise and accurate are repeatable and very close to true values.
What Is Precision In Chemistry
Precision in chemistry is the reproducibility of a measurement. It is also a measure of how close the measurements are to each other. We use this terms, most of the times, for multiple measurements. This term describes how consistent the measurements are when we repeat the experiment. The repeated measurements reduce the random errors. The precision is independent of accuracy.
Recommended Reading: Exponential Growth And Decay Worksheet Algebra 1
How Can I Improve My Accuracy And Precision In Lab Measurements
There are several tricks, depending on what is available at your school.
HOW TO DO BETTER IN LAB
-
Read the lab procedure before coming to lab! It helps you be prepared so that you know what not to do.
-
Label all your bottles, flasks, etc. with some tape if available. That ensures that you don’t mix the wrong substance with something else, and get the wrong observation down.
-
If you feel like you messed up a bit, ask your teacher’s assistant to check, and have them give their input on whether you did it right, if you’re unsure.
-
Leave your glassware far enough away from the edge of your lab station. That helps you to not bump it and spill anything.
HOW TO IMPROVE ACCURACY/PRECISION OF MEASUREMENTS
-
You can use a white piece of paper and wrap it around behind a burette, pipette, graduated cylinder, etc. to read the index mark more clearly. If you use a dark reagent, try to find a burette with light markings.
-
Try to practice doing more precise motions. Practice squeezing DI water bottles more lightly to dispense drop by drop if needed, tilt glassware slowly to pour, use a glass rod to force liquids to travel along them into other glassware, spin the stopcock on a burette #360^@# quickly for a half/quarter-drop, etc.
-
A static gun allows you to get rid of the static electricity around a scale, which stops it from drifting. Scale measurements can drift when you bring blue nitrile gloves near them, which makes it hard to settle on a number.