Periods Groups And The Periodic Table
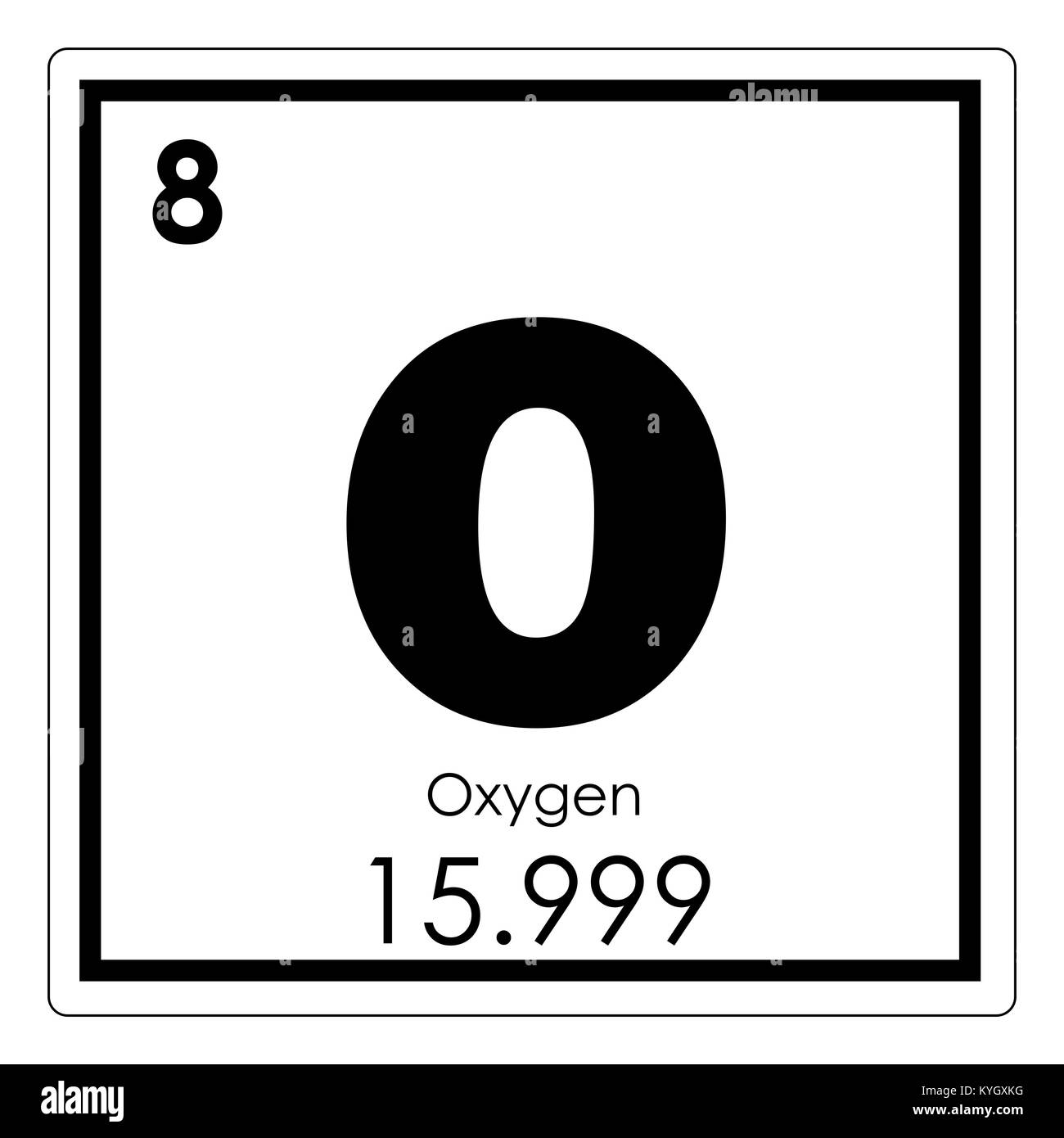
The periodic table is a commonly used method of organizing the elements that provides useful information about the elements and their behavior. In Fig. 2.12, elements in blue are metals and elements in yellow are nonmetals. In Figure 2.13, the entry for hydrogen highlights the placement of the atomic number, element symbol, element name, and atomic weight.
The periodic table has three prominent features. First, the periodic table is arranged in horizontal rows, which are called periods. There are seven periods. In Period 1 there are two elements, hydrogen and helium . The second and third periods both contain eight elements, the fourth and fifth periods contain 18 elements, and the sixth and seventh periods contain 32 elements.
Second, all of the elements are listed sequentially according to their atomic numbers. The atomic number corresponds to the number of protons and is found above the elements symbol. For example, in Figure 2.13, the atomic number of hydrogen is 1, found over the H.
Why Is Hydroxide Negatively Charged
In this compound, two electrons share oxygen bonds with hydrogen. Hydroxide bears a negative charge since an electron has been absorbed. Oxygen, depicted as O, is bound to a hydrogen, depicted as H, so we can see that the negative sign is the most negative component of the product.
Learn more about the different applications, structure, and properties of Hydroxide from the expert faculties at BYJUS Indias largest education company.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Chemical Properties And Reactions
The large values of the electronegativity and the electron affinity of oxygen are typical of elements that show only nonmetallic behaviour. In all of its compounds, oxygen assumes a negative oxidation state as is expected from the two half-filled outer orbitals. When these orbitals are filled by electron transfer, the oxide ion O2 is created. In peroxides it is assumed that each oxygen has a charge of 1. This property of accepting electrons by complete or partial transfer defines an oxidizing agent. When such an agent reacts with an electron-donating substance, its own oxidation state is lowered. The change , from the zero to the 2 state in the case of oxygen, is called a reduction. Oxygen may be thought of as the original oxidizing agent, the nomenclature used to describe oxidation and reduction being based upon this behaviour typical of oxygen.
As described in the section on allotropy, oxygen forms the diatomic species, O2, under normal conditions and, as well, the triatomic species ozone, O3. There is some evidence for a very unstable tetratomic species, O4. In the molecular diatomic form there are two unpaired electrons that lie in antibonding orbitals. The paramagnetic behaviour of oxygen confirms the presence of such electrons.
The intense reactivity of ozone is sometimes explained by suggesting that one of the three oxygen atoms is in an atomic state on reacting, this atom is dissociated from the O3 molecule, leaving molecular oxygen.
Recommended Reading: What Are The 4 Major Goals Of Psychology
Isotopes And Stellar Origin
Naturally occurring oxygen is composed of three stable isotopes, 16O, 17O, and 18O, with 16O being the most abundant .
Most 16O is synthesized at the end of the helium fusion process in massive stars but some is made in the neon burning process.17O is primarily made by the burning of hydrogen into helium during the CNO cycle, making it a common isotope in the hydrogen burning zones of stars. Most 18O is produced when 14N captures a 4He nucleus, making 18O common in the helium-rich zones of evolved, massive stars.
Fourteen radioisotopes have been characterized. The most stable are 15O with a half-life of 122.24 seconds and 14O with a half-life of 70.606 seconds. All of the remaining radioactive isotopes have half-lives that are less than 27 s and the majority of these have half-lives that are less than 83 milliseconds. The most common of the isotopes lighter than 16O is + decay to yield nitrogen, and the most common mode for the isotopes heavier than 18O is beta decay to yield fluorine.
The Best Chemistry O Level Notes
The Best Chemistry O Level Notes compiled from all around the world at one place for your ease.
The Best Chemistry O Level Notes compiled from all around the world at one place for your ease so you can prepare for your tests and examinations with the satisfaction that you have the best resources available to you.
About Chemistry:
Chemistry is the branch of science that deals with the identification of the substances of which matter is composed the investigation of their properties and the ways in which they interact, combine, and change and the use of these processes to form new substances.
Get Chemistry O Level Notes here at my new website.
I hope you find them useful. If you have extra notes or resources please contribute to the website and help thousands of other people like you. In addition, your name will be written in the credits section of this post.
Recommended Reading: H2o2 Lewis Structure Molecular Geometry
Chemistry End Of Chapter Exercises
caffeine, C8H10N4O2
ascorbic acid , C6H8O6
acetic acid, C2H4O2
Life Support And Recreational Use
An application of O2 as a low-pressure breathing gas is in modern space suits, which surround their occupant’s body with the breathing gas. These devices use nearly pure oxygen at about one-third normal pressure, resulting in a normal blood partial pressure of O2. This trade-off of higher oxygen concentration for lower pressure is needed to maintain suit flexibility.
Scuba and surface-suppliedunderwater divers and submariners also rely on artificially delivered O2. Submarines, submersibles and atmospheric diving suits usually operate at normal atmospheric pressure. Breathing air is scrubbed of carbon dioxide by chemical extraction and oxygen is replaced to maintain a constant partial pressure. Ambient pressure divers breathe air or gas mixtures with an oxygen fraction suited to the operating depth. Pure or nearly pure O2 use in diving at pressures higher than atmospheric is usually limited to rebreathers, or at relatively shallow depths , or medical treatment in recompression chambers at pressures up to 2.8 bar, where acute oxygen toxicity can be managed without the risk of drowning. Deeper diving requires significant dilution of O2 with other gases, such as nitrogen or helium, to prevent oxygen toxicity.
Other recreational uses that do not involve breathing include pyrotechnic applications, such as George Goble‘s five-second ignition of barbecue grills.
Also Check: What Is Psychological Foundation Of Education
Health Effects Of Oxygen
Oxygen is essential for all forms of life since it is a constituent of DNA and almost all other biologically important compounds. Is it even more drammatically essential, in that animals must have minute by minute supply of the gas in order to survive. Oxygen in the lungs is picked up by the iron atom at the center of hemoglobin in the blood and thereby transported to where it is needed.
Every human being needs oxygen to breathe, but as in so many cases too much is not good. If one is exposed to large amounts of oxygen for a long time, lung damage can occur. Breathing 50-100% oxygen at normal pressure over a prolonged period causes lung damage. Those people who work with frequent or potentially high exposures to pure oxygen, should take lung function tests before beginning employment and after that. Oxygen is usually stored under very low temperatures and therefore one should wear special clothes to prevent the freezing of body tissues.
New Elements And Compounds
In addition to his joint recognition for the discovery of oxygen, Scheele is argued to have been the first to discover other chemical elements such as , , , and , as well as several chemical compounds, including ,,, ,, and . In addition, he discovered a process similar to , along with a means of mass-producing , leading Sweden to become one of the world’s leading producers of .
Scheele made one other very important scientific discovery in 1774, arguably more revolutionary than his isolation of oxygen. He identified , , and in a specimen of given to him by his friend, , but could not identify an additional component . When he treated the pyrolusite with over a warm sand bath, a yellow-green gas with a strong odor was produced. He found that the gas sank to the bottom of an open bottle and was denser than ordinary air. He also noted that the gas was not soluble in water. It turned corks a yellow color and removed all color from wet, blue litmus paper and some flowers. He called this gas with bleaching abilities, “dephlogisticated muriatic acid” . Eventually, Sir named the gas , with reference to its pale green colour.
Chlorine’s bleaching properties were eventually turned into an industry by , and became the foundation of a second industry of disinfection and deodorization of putrefied tissue and wounds in the hands of , by 1824.
Read Also: What Is Coplanar In Geometry
Biological Production And Role Of O2
In nature, free oxygen is produced by the light-driven splitting of water during oxygenic . According to some estimates, green algae and cyanobacteria in marine environments provide about 70% of the free oxygen produced on Earth, and the rest is produced by terrestrial plants. Other estimates of the oceanic contribution to atmospheric oxygen are higher, while some estimates are lower, suggesting oceans produce ~45% of Earth’s atmospheric oxygen each year.
A simplified overall formula for photosynthesis is
- 6 CO2 + 6 H
2diffuses through membranes in the lungs and into red blood cells. Hemoglobin binds O2, changing color from bluish red to bright red . Other animals use hemocyanin or hemerythrin . A liter of blood can dissolve 200 cm3 of O2.
Until the discovery of anaerobicmetazoa, oxygen was thought to be a requirement for all complex life.
2) and hydrogen peroxide , are reactive by-products of oxygen use in organisms. Parts of the immune system of higher organisms create peroxide, superoxide, and singlet oxygen to destroy invading microbes. Reactive oxygen species also play an important role in the hypersensitive response of plants against pathogen attack. Oxygen is damaging to obligately anaerobic organisms, which were the dominant form of early life on Earth until O2 began to accumulate in the atmosphere about 2.5 billion years ago during the Great Oxygenation Event, about a billion years after the first appearance of these organisms.
Properties And Molecular Structure
At standard temperature and pressure, oxygen is a colorless, odorless, and tasteless gas with the molecular formulaO2, referred to as dioxygen.
As dioxygen, two oxygen atoms are chemically bound to each other. The bond can be variously described based on level of theory, but is reasonably and simply described as a covalent double bond that results from the filling of molecular orbitals formed from the atomic orbitals of the individual oxygen atoms, the filling of which results in a bond order of two. More specifically, the double bond is the result of sequential, low-to-high energy, or Aufbau, filling of orbitals, and the resulting cancellation of contributions from the 2s electrons, after sequential filling of the low and * orbitals overlap of the two atomic 2p orbitals that lie along the OO molecular axis and overlap of two pairs of atomic 2p orbitals perpendicular to the OO molecular axis, and then cancellation of contributions from the remaining two 2p electrons after their partial filling of the * orbitals.
Also Check: How To Calculate Mass In Chemistry
Commercial Production And Use
When required in tonnage quantities, oxygen is prepared by the fractional distillation of liquid air. Of the main components of air, oxygen has the highest boiling point and therefore is less volatile than nitrogen and argon. The process takes advantage of the fact that when a compressed gas is allowed to expand, it cools. Major steps in the operation include the following: Air is filtered to remove particulates moisture and carbon dioxide are removed by absorption in alkali the air is compressed and the heat of compression removed by ordinary cooling procedures the compressed and cooled air is passed into coils contained in a chamber a portion of the compressed air is allowed to expand in the chamber, cooling the coils the expanded gas is returned to the compressor with multiple subsequent expansion and compression steps resulting finally in liquefaction of the compressed air at a temperature of 196 °C the liquid air is allowed to warm to distill first the light rare gases, then the nitrogen, leaving liquid oxygen. Multiple fractionations will produce a product pure enough for most industrial purposes.
What Is Negative Delta H
When enthalpy is negative and delta H is less than zero this means that a system released heat. This is called an exothermic reaction. For example when water changes from liquid to gas delta H is positive the water gains heat. When water changes from liquid to solid delta H is negative the water loses heat.
Recommended Reading: What Is Vx In Physics
Is Negative Delta G Exothermic
Thus it is like an exothermic reaction with a negative value of DE or DH. A reaction with a negative DG is called exergonic to emphasize this. This is an endothermic reaction with a positive entropy change. This sort of reaction is reactant-favored at low temperatures and product-favored at high temperatures.
Magnetic Properties Of Oxygen
As shown, there are two unpaired electrons which causes O2 to be paramagnetic. There are also eight valence electrons in the bonding orbitals and four in antibonding orbitals which makes the bond order 2. This accounts for the double covalent bond that is present in O2.
Video \: A chemical demonstration of the paramagnetism of molecular oxygen, as shown by the attraction of liquid oxygen to magnets.
As shown in Video \, since molecular oxygen has unpaired electrons, it is paramagnetic and is attracted to the magnet. In contrast, molecular nitrogen ) has no unpaired electrons and it not attracted to the magnet.
Recommended Reading: Coryxkenshin Plays Geometry Dash World
Existing Theories Before Scheele
By the time he was a teenager, Scheele had learned the dominant theory of gases which in the 1770s was the phlogiston theory. Phlogiston, classified as “matter of fire”, was supposed to be released from any burning material, and when it was exhausted, combustion would stop. When Scheele discovered he called it “fire air” as it supported combustion. Scheele explained oxygen using phlogistical terms because he did not believe that his discovery disproved the phlogiston theory.
Before Scheele made his discovery of oxygen, he studied air. was thought to be an element that made up the environment in which took place but did not interfere with the reactions. Scheele’s investigation of air enabled him to conclude that air was a mixture of “fire air” and “foul air ” in other words, a mixture of two gases. Scheele performed numerous experiments in which he heated substances such as saltpetre , , heavy metal nitrates, and . In all of these experiments, he isolated the same gas: his “fire air,” which he believed combined with phlogiston in materials to be released during heat-releasing reactions.
Read A Brief Summary Of This Topic
chemistry, the science that deals with the properties, composition, and structure of substances , the transformations they undergo, and the energy that is released or absorbed during these processes. Every substance, whether naturally occurring or artificially produced, consists of one or more of the hundred-odd species of atoms that have been identified as elements. Although these atoms, in turn, are composed of more elementary particles, they are the basic building blocks of chemical substances there is no quantity of oxygen, mercury, or gold, for example, smaller than an atom of that substance. Chemistry, therefore, is concerned not with the subatomic domain but with the properties of atoms and the laws governing their combinations and how the knowledge of these properties can be used to achieve specific purposes.
Recommended Reading: What Does Transversal Mean In Geometry
One Of Several Ways Chemistry Can Be Divided Into Categories
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
There are many branches of chemistry or chemistry disciplines. The five main branches are organic chemistry, inorganic chemistry, analytical chemistry, physical chemistry, and biochemistry.
The Theory Of Phlogiston
Chemical Treatise on Air and Fire
Scheele achieved astonishingly prolific and important results without the expensive laboratory equipment to which his Parisian contemporary Antoine Lavoisier was accustomed. Through the studies of Lavoisier, Priestley, Scheele, and others, was made a standardized field with consistent procedures. Although Scheele was unable to grasp the significance of his discovery of the substance that Lavoisier later named oxygen, his work was essential for the abandonment of the long-held theory of phlogiston.
Scheele’s study of the gas not yet named oxygen was prompted by a complaint by , a professor at who would eventually become Scheele’s friend. Bergman informed Scheele that the saltpeter he had purchased from Scheele’s employer, after long heating, produced red vapors when it came into contact with acetic acid. Scheele’s quick explanation was that the saltpeter had absorbed phlogiston with the heat and gave off a new phlogisticated gas as an active principle when combined with an acid .
When other chemists later showed water is produced when burning hydrogen and that rusting of metals added weight to them and that passing water over hot iron gave hydrogen, Scheele modified his theory to suggest that oxygen was the salt , and that when added to iron, water was reproduced, which added weight to the iron as rust.
You May Like: What Does S Mean In Physics