Three Types Of Osmotic Solutions
There are three types of solutions isotonic, hypertonic and hypotonic solutions in which osmosis plays a key role and occurs differently understanding these basic examples is necessary before learning about the cool and more complex details of osmosis, as well as its importance on so many aspects of our survival.
Iiia Definition Of Osmosis
Osmosis refers to the movement of fluid across a membrane in response to differing concentrations of solutes on the two sides of the membrane. Osmosis has been used since antiquity to preserve foods by dehydration with salt or sugar. The removal of water from a tissue by salt was referred to as imbibition. This description comes from the notion that these solutes attracted water from material they touched. In 1748, J.A. Nollet used an animal bladder to separate chambers containing water and wine. He noted that the volume in the wine chamber increased and, if this chamber was closed, a pressure developed. He named the phenomenon osmosis from the Greek o, meaning thrust or impulse.
FIGURE 16.2. Plot of data from Pfeffer for the osmotic pressure of sucrose solutions. A copper ferrocyanide precipitation membrane was formed in the walls of an unglazed porcelain cup. The membrane separated a sucrose solution in the inner chamber from water in the outer chamber. The inner chamber was then attached to a manometer and sealed. The linear relation between the pressure measured with this device and the sucrose concentration were the experimental impetus for deriving vant Hoffs law.
Tiree Withers, Simon P. Neill, in, 2021
Diffusion And Osmosis Worksheet Key Diffusion Osmosis
The biology of osmosis jones answer key. Osmosis jones worksheet answer key osmosis and tonicity worksheet answer key and osmosis jones worksheet answer key are three main things we want to show you based on the gallery title. Worksheets are diffusion and osmosis work answers the biology of osmosis jones. 31 Osmosis Jones Worksheet Answer Key Free.
Osmosis jones worksheet answers related files Osmosis jones questions released 2001 help keep students engaged throughout the film by providing 28 questions for them to answer to keep them on track. Jones this worksheet along with the movie osmosis. What does Ozzies bullet hit causing f.
The biology of osmosis. 29 The Biology Of Osmosis Jones Worksheet Answer Key. Biology Of Osmosis Jones Worksheet Answer Key.
Start studying osmosis jones answer key. Https Static1 Squarespace Com Static 606148317a82692d704ec529 T 606188c51052d72d0414923d 1617004743826 44882129554 Pdf. White blood cell 2.
This worksheet and answer key will help teach your students the biology behind Osmosis Jones. A cop and his girlfriend are going to the kidneys to see who. Jonesto reinforce concepts about the human body.
Diffusion diffusion is a. Pages275 words Check Price. A cop and his girlfriend are going to the kidneys to see who.
Osmosis_Jones_Worksheetpdf Read File Online Report Abuse. Osmosis Foldable Inside Cover Biology Teaching Biology Science Cells. Biology Diffusion And Osmosis Worksheet Answer Key Worksheet.
Pin On My Board
You May Like: Formula For Volume Physics
Osmosis Applications And Uses
We asked, what is osmosis in biology, and a logical follow-up question is, what are the applications of osmosis?
Another easy osmosis experiment to try at home:
You need two glass or ceramic cereal-sized bowls, one large carrot or two baby carrots, salt, and water.
We can see something interesting when we drop a carrot into a bowl of saltwater. Within hours the carrot will have become a limp, orange piece of ribbon.
Why? Because the water left the carrot to balance the high concentration of salt surrounding the carrot.
Have you ever watched a suspense movie where the stranded travelers on a desert island are longing for something to drink and one wise traveler warns the others, Do not drink the ocean water! A diet of ocean water would leave your cells void of water as it traveled to counteract the salt.
Remove The Shell From Two Eggs
Place two egg in a container of vinegar for about 24 hours. The eggs should be completely submerged.
After about 24 hours, removed the eggs and gently rub the shell under cold running water. You should be able to remove most of the shell.
If it wont all rub off put the eggs in fresh vinegar for another few hours.
Don’t Miss: Ccl4 Geometric Shape
Water Potential/ Solvent Potential
- The water potential across a semi-permeable membrane also influences the rate of osmosis.
- As the water potential of a solution is more, the water molecules can move across the membrane as the pressure exerted by the particles is increased.
- Eventually, the water potential on either side becomes equal, creating equilibrium.
- Once equilibrium is reached, water continues to flow across the membrane, but it flows both ways in equal amounts, thus stabilizing the solutions.
Teaching Osmosis To Biology Students
Arthur Louis Odom, Lloyd H. Barrow, William L. Romine Teaching Osmosis to Biology Students. The American Biology Teacher 1 August 2017 79 : 473479. doi:
Osmosis is a fundamental concept of great importance to understanding natural biological, physical, and chemical processes. We provide an instructional guide to assist instructors of advanced high school biology and college biology students in defining questions that are central to deriving a highly developed understanding of osmosis. We present teaching activities that focus on advancing multiple hypotheses about the cause of osmosis, presenting a tentative explanation and model of osmosis, and drawing scientifically accepted conclusions about osmotic processes.
Don’t Miss: Who Coined The Word Geography
Osmosis Facts For Kids
Here are some interesting facts about osmosis:
# Osmosis can be observed in the working of specific organs, notably the kidneys. The kidneys job is to filter waste items from the bloodstream and expel them in the form of urine. More than a million microfilters called nephrons are found in each kidney, allowing microscopic particles like water, glucose, urea, and ions to flow through while keeping blood molecules out.
After this filtration, the kidneys must reabsorb enough water to keep the blood plasma in a healthy equilibrium through osmosis.
# Hypotonic solutions have a lower concentration of solutes outside the cell hence, water permeates the cell, which gains volume. A hypertonic has a higher solute concentration than inside the cell, and the solutes cannot cross the membrane.
# A partly permeable membrane allows water to pass through it. Larger molecules, such as sugar are unable to pass through it.
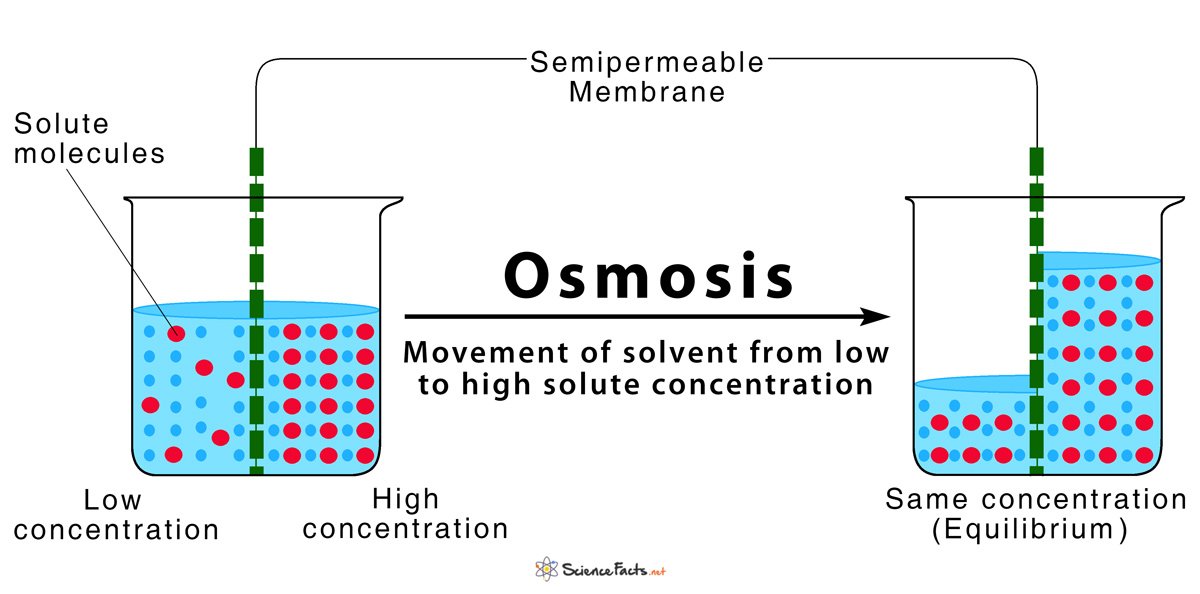
# Water travels from a dilute solution to a concentrated solution .
# When too much water enters a cell through osmosis in living things, it expands. Animal cells are prone to rupture due to heavy expansion.
Learning courses for your kids! Get free trial here
The Biology Of Osmosis Jones Answer Key
View homework help osmosis jones biology worksheet answers207793png from science 0899 at denison high school. The biology of osmosis jones answer key PDF Free Download Worksheets are name date period osmosis practice problems lab 5 osmosis tonicity and diffusion and osmosis work answers osmosis work 20 points.
Force And Motion Worksheet Answers Newton S Laws Motion Worksheet
You May Like: Geometry Worksheet Answers Mcdougal Littell
Osmosis In Living Cells
Osmosis has a different effect on the cells. In comparison to a plant cell, an animal cell will lyse when placed in a hypotonic solution, but due to the fact that the plant cells walls are thick, it requires more water. Hence, when placed in a hypotonic solution, the cells will not burst. In reality, a plant cell flourishes in a hypotonic solution. Only an isotonic solution can support an animal cell. Plant cells become turgid and the plants leaves droop in an isotonic solution.
What Is Osmosis In Biology Understanding How Solvents Break The Barrier
Are you getting ready for your first biology class? Or are you trying to shake off the cobwebs and remember your biology from years ago? Either way, you may be asking, what is osmosis in biology?
We want to answer this question in a way that is thorough and understandable at the same time. Dust off your old textbook and put on your reading glasses as you find answers to the question, What is osmosis in biology?
You May Like: Write The Segment Addition Postulate For The Points Described
Types Of Osmotic Solutions
There are three different types of solutions that exist when it comes to osmosis. They are as follows
When describing whether a solution will cause water to move into or out of a cell, three terms are used: hypertonic, hypotonic, and isotonic.
When a cell is placed in a hypertonic solution, it will lose volume due to a net flow of water out of the cell. If the concentration of solutes in a solution is higher than that inside the cell, and the solutes cannot cross the membrane, the solution is hypertonic to the cell.
However, when a cell is placed in a hypotonic solution, it will gain volume due to a net flow of water into the cell. The solution is hypotonic to the cell if the solute concentration outside the cell is lower than inside the cell and the solutes cannot cross the membrane.
Furthermore, when a cell is submerged in an isotonic solution, there is no net flow of water into or out of it, and the volume of the cell remains constant. The solution is isotonic to the cell if the solute concentration outside the cell is the same as inside the cell and the solutes cannot cross the membrane.
Practical Importance Of Osmosis
Now that you understand the basic processes of osmosis, and what different conditions will cause osmosis to occur, you will be able to see the value of this process in so many areas for every form of life.
For plants, osmosis is responsible for the movement of water into the root system, which allows the plant to grow and survive. The root hairs of plants are the key point where minerals and water are taken into the organism. The concentration of water molecules are less in the root hairs than in the soil , so water moves into the cells of the root hairs osmosis continues through numerous layers of cells until that water reaches the xylem tubes equivalent to human veins.
On a related note, when water is taken into the cells of plants, the pressure caused by that osmotic movement is called turgidity. When equilibrium is achieved, those plant cells should be full of water, as well as firm and turgid. This prevents leaves from wilting, allowing them to increase their surface area for sunlight capture. Osmosis also helps protect plants against drought and frost damage, as well as in regulating the opening and closing of stomata.
Don’t Miss: Michael Jackson Biological Kids
The Other Side Of The Coin
Remember the folks on the desert island? While osmosis could lead to their death through the consumption of saltwater, osmosis could also be their best friend. Since osmosis is a two-way street, it flows into and out of cells depending on concentration levels, it can actually be used to turn saltwater into something salt-free and drinkable.
While the stranded folks wouldnt have the proper tools to reverse osmosis on the desert island, it is not impossible for someone with an understanding of science and osmosis.
Basically, the pressure is created to push water from highly concentrated areas into an area away from the salt. Today, small units can actually be purchased to reverse osmosis and create safe drinking water.
Heres an example of a large unit, used in Australia, to clean saltwater for drinking:
The Significance Of Osmosis:
-
Osmosis plays an important role in the transportation of nutrients and the release of metabolic waste products within a living cell.
-
It stabilizes the internal movement of water and intracellular fluid levels within a cell.
-
Osmosis also controls cell-to-cell diffusion and maintains the mechanical structure of a cell.
-
In plants, growing root tips remain turgid and can penetrate easily into the soil because of osmosis.
-
Osmosis plays a major role in the germination of seeds.
Examples of Osmosis
-
The absorption of water by plant roots from the soil.
-
The guard cells of a plant cell are affected by osmosis. When a plant cell is filled with water the guard cells swell up for the stomata to open and let out excess water
-
If you keep your fingers in water for a long time, they become prunes. The reason behind this is that the skin absorbs water and expands.
Don’t Miss: Punchline Bridge To Algebra Did You Hear About
How Osmosis Affects Cells
Osmosis affects plant and animal cells differently because plant and animal cells can tolerate different concentrations of water. In a hypotonic solution, an animal cell will fill with too much water and lyse, or burst open. However, plant cells need more water than animal cells, and will not burst in a hypotonic solution due to their thick cell walls hypotonic solutions are ideal for plant cells. The optimal condition for an animal cell is to be in an isotonic solution, with an equal amount of water and solutes both inside and outside. When a plant cell is in an isotonic solution, its cells are no longer turgid and full of water, and the leaves of the plant will droop. In a hypertonic solution, water will rush out of both animal and plant cells, and the cells will shrivel . This is why slugs and snails shrivel and die when salt is sprinkled onto them water leaves their cells in order to balance the higher concentration of salt outside the cells.
This figure shows the effects of osmosis on red blood cells:
Osmotic Solutions In Living Systems
Before a detailed examination of how osmotic solutions are used in the living systems, it is important to define a key term known as tonicity. Tonicity means that an extracellular solutions ability to cause water to move into or out of a cell via osmosis. Now that a clear definition is given, a deep dive can commence into their effects on a living system.
When a cell is submerged in a hypertonic solution, water escapes, and the cell shrinks, and when then there is no net water movement in an isotonic environment, so the cell size does not change, whereas water will enter a cell when it is placed in a hypotonic environment, causing it to swell.
A hypotonic extracellular solution, on the other hand, is ideal for a plant cell. The plasma membrane can only expand to the rigid cell walls limit, preventing the cell from bursting or lysing. Water will enter a cell until its internal pressure prevents further influx. It is critical for the plants health to maintain this balance of water and solutes. If a plant isnt watered, the extracellular fluid becomes isotonic or hypertonic, causing water to escape from the cells. This causes a drop in turgor pressure, which youve probably noticed as wilting. The cell membrane may detach from the cell wall and constrict the cytoplasm under hypertonic conditions, a condition known as plasmolysis.
Also Check: Is Paris Jackson Michael’s Biological Daughter
What Is Osmosis In Biology
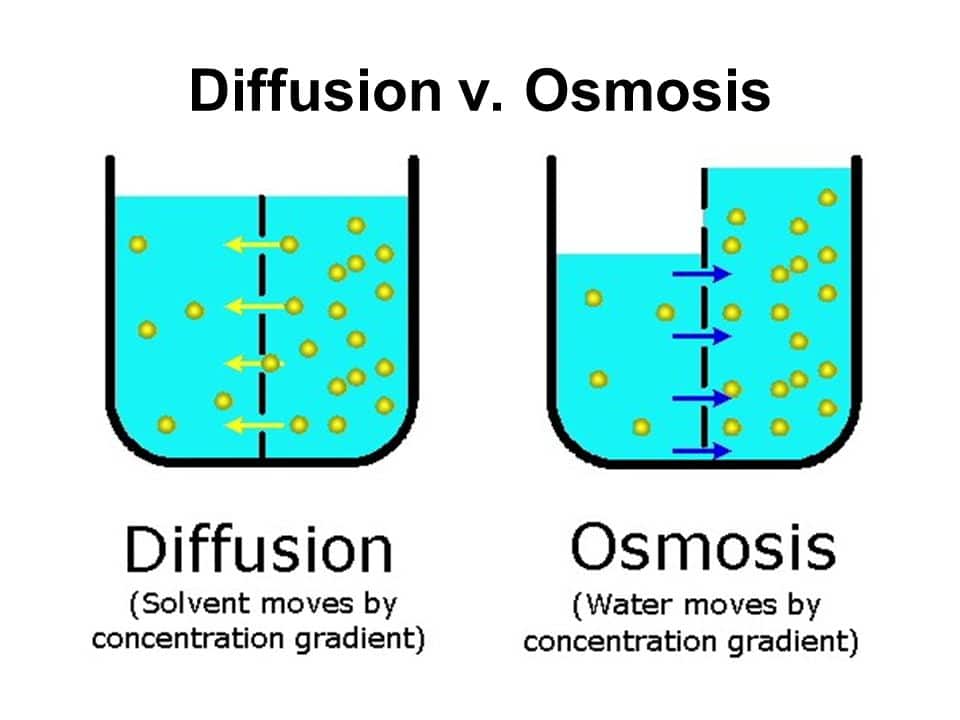
Osmosis is the transport of a solvent through a semipermeable membrane that separates two solutions of differing solute concentration. During osmosis, the solvent moves from the solution that is lower in solute concentration to the solution that is higher in solute concentration.
We carry a lot from school. Especially those who managed to learn the only correct class approach back in Soviet times: what is the very image of a bloodsucking capitalist in a top hat and a cigar in his mouth, sitting high on sacks of dollars, surrounded by proletarians covered in rags with skinny children in their arms And art! How many images of the poor, but honest and noble, and how many nasty rich people, ready for any abomination for the sake of an extra million, are in the space around us. Even the social parasite and idler dArtagnan is much more attractive to us than the bourgeois workers of his day .
chronic lie
Scientists insist that if parents want their children to have a rich vocabulary and well-delivered speech by the beginning of school, it is worth paying special attention to emotional communication with them.
Role In Living Things
Osmotic pressure is the main agent of support in many plants. The osmotic entry of water raises the turgor pressure exerted against the cell wall, until it equals the osmotic pressure, creating a steady state.
When a plant cell is placed in a solution that is hypertonic relative to the cytoplasm, water moves out of the cell and the cell shrinks. In doing so, the cell becomes flaccid. In extreme cases, the cell becomes plasmolyzed the cell membrane disengages with the cell wall due to lack of water pressure on it.
When a plant cell is placed in a solution that is hypotonic relative to the cytoplasm, water moves into the cell and the cell swells to become turgid.
Osmosis is responsible for the ability of plant roots to draw water from the soil. Plants concentrate solutes in their root cells by active transport, and water enters the roots by osmosis. Osmosis is also responsible for controlling the movement of guard cells.
In unusual environments, osmosis can be very harmful to organisms. For example, freshwater and saltwater aquarium fish placed in water of a different salinity than that to which they are adapted to will die quickly, and in the case of saltwater fish, dramatically. Another example of a harmful osmotic effect is the use of table salt to kill leeches and slugs.
Suppose an animal or a plant cell is placed in a solution of sugar or salt in water.
Read Also: What Does Capital G Mean In Physics