Types And Methods Of Dilution
Above, we introduced \
We use this equation when we want to make a fixed amount of a dilute solution from a stock solution.
Within chemistry, a stock solution is usually a large volume of a reagent thats going to be diluted down in concentration for usage.
This type of dilution is usually called a simple dilution which was shown above.
Solution Concentration And Dilution
The relative amount of a given solution component is known as its concentration. Often, though not always, a solution contains one component with a concentration that is significantly greater than that of all other components. This component is called the solvent and may be viewed as the medium in which the other components are dispersed or dissolved. Solutions in which water is the solvent are, of course, very common on our planet. A solution in which water is the solvent is called an aqueous solution.
A solute is a component of a solution that is typically present at a much lower concentration than the solvent. Solute concentrations are often described with qualitative terms such as dilute and concentrated .
Concentrations may be quantitatively assessed using a wide variety of measurement units, each convenient for particular applications. Molarity is a useful concentration unit for many applications in chemistry. Molarity is defined as the number of moles of solute in exactly 1 liter of the solution and has the units of mol/L.
Dilution of Solutions
Dilution is the process whereby a solution is made less concentrated by the addition of solvent. For example, a glass of iced coffee becomes increasingly dilute, and less sweet, as the ice melts. In laboratories, solutions are often stored in their concentrated forms, called stock solutions. Solutions of lower concentrations are prepared from stock through dilution.
How To Perform An Emulsion Dilution Test
The dilution test is based on the principle that an emulsion can be diluted with its continuous phase, diluted and the separation is apparent. in aqueous phase and oil-soluble will be dispersed in the oil phase of an emulsion. An electrical conductivity test is that water conducts an electric current and oils do not.
Don’t Miss: What Kind Of Jobs Can You Get With Psychology Degree
The Formula For Dilution:
In both the dilution and concentration processes, the amount of solute stays the same. As a result, this gives us a way to calculate what the new solution volume must be to get the desired concentration of the solute. From the definition of the molarity we know,
molarity = \
Then we may solve for the number of moles of solute as:
moles of solute = \ \times \)
We represent the molarity by M and volume of solution by V. Therefore, the equation becomes
moles of solute = M V
Since this quantity does not change before and after the change of concentration. Therefore the product MV must be the same before and after the concentration change. Using numbers to represent the initial and final conditions, we will get the dilution equation:
Here, the volumes must be expressed in the same units. Also, this equation gives only the initial and final conditions, not the amount of the change. We may find the amount of change by subtraction.
Where,
| the volume of the diluted solution |
Examples Of Dilution In Chemistry
You are in science class, and you have a stock solution of 30 mL of 1 M NaCl. The teacher tells you to dilute it to 50 mL by adding water. Whats the final concentration after dilution?
The answer is simple, use our simple dilution formula and follow the steps shown below:
Figure 5: Simple Dilution formula. Daniela Lin, Study Smarter Originals.
And we start with a stock solution of 30 mL of 1 M NaCl
So \ 1 M of \ and \ of \ solution
We end up with 50 mL solution which means \ and we’re trying to find \.
Since we’re solving for \ then we can rearrange the simple dilution formula equation to:
Figure 6: Simple dilution formula example. Daniela Lin, Study Smarter Originals.
So our final concentration is 0.6 M for our solution.
Another interesting question to ask might be what’s the amount of solvent we added?
In this case, the answer is simple we just subtract 50 mL by 30 mL and we get 20 mL. This means we added 20 mL of solvent to the solution.
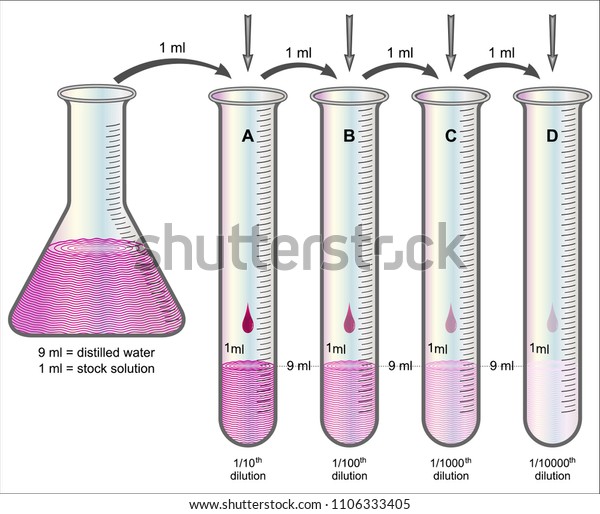
Figure 7 : Serial Dilutions explained. Daniela Lin, Study Smarter Originals.
Serial dilutions are usually performed in the lab. From left to right as shown in the illustration, were diluting down by a 1:10 ratio each time. We find the dilution factor by adding 1 mL sample over 9 mL of diluent plus 1 mL of sample, giving you a 1:10 ratio.
Serial dilutions are performed, usually to avoid having to pipette very tiny amounts of liquid to make a specifically required dilution.
To perform a serial dilution, we just need to:
Don’t Miss: What Is Location In Geography
Procedure Of Serial Dilution
The following is the procedure for a ten-fold dilution of a sample to a dilution factor of 10-6:
Calculating Final Dilution Factor And Concentration
Also Check: What Is Electronic Configuration In Chemistry
Difference Between Dilution And Concentration
May 18, 2011 Posted by Madhu
The key difference between dilution and concentration is that dilution refers to the addition of more solvent whereas concentration refers to the removal of the solvent.
The concepts of dilution and concentration are very significant in the study of solutions in chemistry. Consequently, the amount of solute in a solvent decides the properties of a solution and this amount remaining the same we can make a solution diluted or concentrated by adding solvent and removing some of the solvent from the solution. Thus, in chemical analysis, we need to change the concentration of a solution often in different applications.
What Is The Dilution Factor
After dilution, the dilution factor represents how much of the original stock solution remains in the entire solution. Its usually expressed as a ratio, although it can also be expressed as an exponent.
The part of the stock solution to the part of the dilutant added or the part of the stock solution to the part of the total solution is described by the dilution factor, which can be expressed as a ratio or an exponent.
Because the differences between these two representations are so minor, an example would be helpful:
Lets imagine we have a 10 cm3 acyl chloride aqueous solution. However, because this solution is excessively concentrated for our experiment, we add 90 cm3 of water to dilute it further. We get 100 cm3 of acyl chloride in the end. The S:D ratio is 1:9 since we have 10 parts stock solution and 90 parts dilutant . The dilution factor is 1:10 in S:T notation. Thus we have 10 cm3 of stock solution that now makes up a 100 cm3 solution.
Its also worth noting that dilution factors merely represent a reduction in concentration no molecules are lost, but the number of molecules per mL does. This is beneficial in a variety of experimental circumstances.
Although the dilution factor is merely a convenient method of thinking about dilutions, they are highly widespread in both science and everyday life. Theyre also employed in almost all chemical and biological research because the stock solution of our substance is frequently far more concentrated than you want.
Recommended Reading: What Is Taxonomy In Biology Class 11
Performing A Basic Dilution
What Is The Difference Between Dilution And Concentration
Dilution is the process of decreasing the concentration of solutes in a solution by adding more solvent whereas concentration is the process of increasing the concentration of solutes in a solution. Thus, the key difference between dilution and concentration is that dilution refers to the addition of more solvent whereas concentration refers to the removal of the solvent. We can dilute a solution by either adding more solvent or by removing the solutes while the process of concentration involves either the addition of more solutes or the removal of the solvent.
The below infographic provides further details on the difference between dilution and concentration.
Don’t Miss: What Do Sex Dreams Mean Psychology
Summary Dilution Vs Concentration
Dilution and concentration are very important in chemistry to prepare a solution with the desired concentration. Moreover, these processes of dilution and concentration are very important in analytical chemistry. The key difference between dilution and concentration is that dilution refers to the addition of more solvent whereas concentration refers to the removal of the solvent.
Reference:
1. Dilution . Wikipedia, Wikimedia Foundation, 22 Sept. 2018. Available here
Image Courtesy:
1.DilutionBy Theislikerice Own work, via Commons Wikimedia 2.Dilution-concentration simple exampleBy FirstPrinciples via Commons Wikimedia
Examples Of Standard Dilutions
A standard dilution is a one-time event, mixing the reagent with diluent and using this solution in your analysis.
We frequently dilute things times 2, which means 1 part reagent mixed with 1 part diluent. For example, I will mix 1 mL calcium reagent with 1 mL of deionized water to make a times 2 dilution. This is written as a 1:2 dilution or a times 2 dilution. The first number is the volume of reagent and the second number is the total volume of the final solution . Expressing a x2 dilution as a ratio would be 1:1, or 1 mL reagent plus 1 mL water.
Lets try another example. A medical laboratory scientist must dilute a specimen times 10 with normal saline prior to using it for analysis to ensure the analyte in the specimen is at the proper ratio to interact with the reagent optimally. So, this 1:10 dilution is 1 part specimen to 10 total parts of solution, which if we need 10 mL of solution, equates to 1 mL specimen and 9 mL normal saline. The ratio is 1:9, or 1 mL specimen and 9 mL normal saline.
As you can see in this example, the volume of specimen is the same in the dilution and ratio, and the volume of saline is the same in both expressions, as well. However, the way they are expressed is slightly different. You just need to understand the difference when explaining them. A 1:10 dilution is not the same as a 1:10 ratio. A 1:10 dilution is the same as a 1:9 ratio.
| Term |
| 9 parts 10 parts |
A specimen is tested for cholesterol and diluted 1:10 prior to analysis.
You May Like: What Does Classical Conditioning Mean In Psychology
What Is A Dilution In Chemistry
What is dilution one word answer?Dilution is when something is watered down or weakened.
What is dilution in science?
A common method of making a solution of a given concentration involves taking a more concentration solution and adding water until the desired concentration is reached. This process is known as dilution.
What is another word for dilution?
In this page you can discover 34 synonyms, antonyms, idiomatic expressions, and related words for dilute, like: water-down, thin-out, thin, waterish, weaken, concentrate, reduce, adulterate, alter, cut and diminish.
What is called dilution?
Dilution is the process of lowering the concentration of a solute in a solution by simply adding more solvent to the solution, such as water. Diluting a solution entails adding more solvent without adding more solute.
Chemistry Is Everywhere: Preparing Iv Solutions
In a hospital emergency room, a physician orders an intravenous delivery of 100 mL of 0.5% KCl for a patient suffering from hypokalemia . Does an aide run to a supply cabinet and take out an IV bag containing this concentration of KCl?
Not likely. It is more probable that the aide must make the proper solution from an IV bag of sterile solution and a more concentrated, sterile solution, called a stock solution, of KCl. The aide is expected to use a syringe to draw up some stock solution and inject it into the waiting IV bag and dilute it to the proper concentration. Thus the aide must perform a dilution calculation.
If the stock solution is 10.0% KCl and the final volume and concentration need to be 100 mL and 0.50%, respectively, then it is an easy calculation to determine how much stock solution to use:
Of course, the addition of the stock solution affects the total volume of the diluted solution, but the final concentration is likely close enough even for medical purposes.
Medical and pharmaceutical personnel are constantly dealing with dosages that require concentration measurements and dilutions. It is an important responsibility: calculating the wrong dose can be useless, harmful, or even fatal!
Key Takeaways
- Calculate the new concentration or volume for a dilution or concentration of a solution.
Exercises
Read Also: What Is E Called In Math
How To Calculate Dilution Factor
Below mentioned are the steps to calculate the dilution factor by hand:
- Find any two of the following three values: stock solution volume , dilutant solution volume , and total solution volume . This can be done either theoretically or experimentally .
- With this equation, we can find the third volume using the two volumes: stock + dilutant = total. This step may not be necessary if we know which notation we want to use , but it is included for completeness.
- Convert the numbers to the same units as each other.
- We decide which notation we require:
- S:D = Set the stock and dilutant amount values as a ratio stock:dilutant.
- S:T = Set the stock and total amount values as a ratio stock:total
Problem Solving : Dilution Factor
The Problem:
The site of a meteorite which fell to Earth millions of years ago is suspected of having enough iron in it to make it worthwhile trying to mine it.Jo the Geologist has taken a sample of the meteorite to Chris the Chemist to determine how much iron is present.Chris has dissolved 10.00 grams of the meteorite sample in 5.00 L of acid to produce a stock solution. Chris ran a trial of the analytical procedure on the stock solution, but found the solution was too concentrated to be used.So Chris prepared a 1:250 dilution of the sample solution, and, after running the analytical procedure successfully, found the solution contained 1.62 x 10-5 mol L-1 Fe2+.What is the mass of iron in the meteorite sample?
Solving the Problem
Using the StoPGoPS model for problem solving:
| STOP! |
Recommended Reading: What Jobs Require Biology Degree