Lewis Structure Of H2o
The Lewis structure of hydrogen and 2 oxygen atoms shows a total of eight valence electrons participate in the bond formation to form a single triatomic H2O molecule.
Here, we need to understand how the Lewis structure is drawn for the H2O molecule:
H2o Lewis Structure Molecular Geometry And Hybridization
H2O is the molecular formula of water, one of the major constituents of the Earth. A single molecule is made up of two hydrogen atoms and one oxygen atom, which are bonded through the covalent bond. Moreover, two or more H2O molecules connect with the help of hydrogen bonds to form a compound.
It is interesting to realize that the covalent bonds are stronger than the hydrogen bonds, that is the reason why water readily reacts with the majority of the chemical elements from the periodic table.
The Lewis structure, or also called an electron dot structure, is a diagrammatic representation of determining the total number of valence electrons present in an atom, which are ready to undergo bond formation to form a molecule and ultimately a compound.
The valence electrons are shown by drawing them as dots around the symbol of the atom, mostly in pairs.
The maximum number of dots that can be drawn is eight per atom, as per the octet rule. Moreover, the formation of a bond because of reacting valence electrons are shown with the help of the lines.
The atomic number of a hydrogen atom is one, which makes its electronic configuration 1s1. As the 1s shell can accommodate a maximum of two electrons, there is a dearth of one more electron.
It makes a single hydrogen atom to have one valence electron.
Besides this, in the case of oxygen, its electronic configuration is 1s2 2s2 2p4 where 2p shell can accommodate six electrons.
Count Total Valence Electron In H2o2
In the first step, we need to calculate how many valence electrons are present in it. So, for this look at the periodic group of hydrogen and oxygen.
As hydrogen has only one electron in its valence shell and oxygen belongs to the 16th group in the periodic table so it has 6 electrons in its valence shell.
Valence electron of Oxygen = 6
Valence electron of Hydrogen = 1
Total valence electron available for drawing the H2O2 lewis structure = 1*2 + 2*6 = 14 valence electrons
Recommended Reading: How To Calculate Half Life Of A Reaction
Co2 Lewis Structure And Molecular Geometry
Carbon dioxide is a colourless, odourless, incombustible gas resulting from the oxidation of carbon. Its Lewis structure comprises two different atoms: carbon, and oxygen. It is a nonpolar molecule with bond angles of 180 degrees. CO2 is used as the refrigerant in fire extinguishers and it is a significant greenhouse gas in Earths atmosphere.
| Name of molecule | |
| No of Valence Electrons in the molecule | 16 |
Check The Stability And Minimize Charges On Atoms By Converting Lone Pairs To Bonds
If molecule or ion contains so many charges on atoms, that structure is not stable. If we got a such structure, we should try to minimize charges by converting lone pairs to bonds.
Because there is no charges on atoms, no need to reduce charges as a step of drawing best lewis structure. Already, we got the best lewis structure for H2O.
Questions
Don’t Miss: Algebra And Trigonometry 4th Edition Stewart
Complete Central Atom Octet And Make Covalent Bond If Necessary
This is the final step for drawing the H2O2 lewis structure. In this step place those remaining valence electrons around oxygen atoms and complete its octet rule.
As we have a total of 8 valence electrons remaining and oxygen needs 8 electrons to complete its outer shell. Also, each oxygen shares 4 electrons already with the help of single bonds in between them.
Hence, each oxygen needs only 4 valence electrons around them for completing their octets. So, put the remaining valence electrons on the oxygen atom.
What Is A Carbon Footprint
A carbon footprint, according to the World Health Organization, is a measurement of the influence of our activities on the earths natural greenhouse. Carbon emissions are mostly caused by human activities such as the burning of fossil fuels, deforestation, and land-use changes, which result in an increase in greenhouse gas concentrations in the atmosphere. To know more, check the article How to Reduce Carbon footprint.
Don’t Miss: Ccl4 Electron Geometry
What Is The Bond Angle Of Ch2o
Every atom in the CH2O molecule has a bond angle of 120º with respect to the central atom because the central atom is surrounded by three regions of electron density and according to the VSEPR theory, these electron cloud needs to be far as possible to avoid the repulsive force.
The electron cloud formed the plane where the central atom lies in the center of the triangle and other atoms are at the corner of the triangle. After, the band will emerge from the central atom at angles of about 120° to each other.
The actual bond angles in the CH2O molecule are HCH = 116 ° and HCO = 122 °.
Two Factors That Indicate The Polarity Of Ch2o
1. Electronegativity:
Electronegativity means the tendency of an atom to attracting electrons towards itself.
If the electronegativity difference between the atoms is high then the polarity will also be higher. Now, look at the electronegativity of carbon and oxygen.
As carbon electronegativity is around 2.6 and for oxygen, it is around 3.45. Therefore, oxygen has a higher tendency to attract an electron to itself than carbon.
Also, the electronegativity difference between carbon and oxygen is more than 0.5, and according to the Pauling scale if the electronegativity difference between atoms is higher than 0.5 then the bond between that atoms behaves as polar.
2. Dipole moment:
Dipole moment ensures the strength of polarity between carbon and oxygen atom. As greater the dipole moment of the molecule, the more is the polar nature of that molecule. The electronegativity difference between the atoms induced positive and negative charges.
As oxygen is more electronegative than carbon, hence, some negative charge is induced on the oxygen atom and a partial positive charge is induced on the carbon atom.
The inducing of these positive and negative charges generates the dipole moment directed from carbon to oxygen atom because oxygen atom is more electronegative than a carbon atom, so, it attracts more electrons towards itself.
The net dipole moment of formaldehyde is 2.330 D.
In mathematical terms, we can express dipole moment as D = Q×R
Read Also: Holt Geometry Chapter 7 Test Form A Answers
Hydrogen Peroxide Lewis Structure
Molecular Geometry Of H2o2
We employ Valence Shell Electron Pair Repulsion to determine the molecular geometry of H2O2.
VSEPR theory states that the molecules adopt a geometry to minimize the repulsion between the electron clouds.
These interactions are of three kinds. In descending order of strength, they are as follows:
1. Lone pair Lone pair repulsion
2. Bond pair Bond pair repulsion
3. Bond pair Bond pair repulsion
We shall use the VSEPR geometry table to determine the geometry.
Here, A is the central atom, X is the substituent, and E is the electron lone pair.
The table is valid only for molecules with a central atom . However, we can approximate a part of the H2O2 molecule as an AX2E2 type molecule.
We write H-O as R. Thus, the formula for H2O2 becomes R-O-H. Now, the VSEPR table can be applied. The molecule is of AX2E2 type with two bond pairs and two lone pairs.
Thus, the geometry will be of bent type. But, we have applied the VSEPR model to only one of the O atoms. Having bent geometry at each O atom results in an open book structure for the H2O2 molecule.
Note: The bond lengths and angles differ slightly in the solid crystalline phase and the figure shown below . The reasons for this are out of scope for this article. However, the open book structure remains the same.
Recommended Reading: Who Is Khloe Kardashian’s Real Father
Why The Double Bond Is Formed Between Carbon And Oxygen Atom In The Ch2o Lewis Structure
This is because the carbon needs 8 electrons in its outer shell to complete the octet and attains stability, hence, for completing its octet, we converted one lone pair of oxygen atoms to a single covalent bond.
Therefore, the bond formed between carbon and oxygen in the CH2O lewis structure is a double covalent bond in nature.
Also, the formal charge formed on double-bonded carbon and oxygen atom is completely neutralized, hence, it ensures the stability of the CH2O lewis structure.
Why Are The Electron And Molecular Geometry Of Ch2o Is Same
Electron geometry predicts the geometry of a molecule with the help of lone pair as well bond pair but molecular geometry has only taken bond pair to predict the shape of the molecule.
As we know, there is no lone pair present on the central atom in the CH2O molecule, hence, the prediction of the geometry is only done on the basis of bonded pair around the central atom.
Therefore, both electron and molecular geometry of CH2O is trigonal planar in nature.
Read Also: Paris Jackson Biological
Complete Octet Of The Molecule
To complete the of the molecule, the carbon atom will donate its electrons to both Oxygen atoms to form a double bond. The carbon atom will donate its electrons to Oxygen atoms as they are more electronegative. Now that you know how the Carbon dioxide Lewis structure is drawn, now let us quickly look at the CO2 molecular geometry.
How Many Shared Pair Or Lone Pair Electrons Are Present In The Ch2o Lewis Structure
As per CH2O lewis structure, the carbon central atom is attached with two single bonds and one double bond, hence, the number of shared pair electrons are = 8 shared pair of electrons.
Also, there are only four lone pairs of electrons are present on oxygen atoms and zero on the carbon and hydrogen atoms in the CH2O lewis structure.
Recommended Reading: Glencoe Geometry Worksheets
S Of Drawing Lewis Structure Of H2o
There are some steps to follow to draw a lewis structure properly. For H2O molecule, its lewis structure and those steps are explained in detail in this tutorial. Because water molecule is simple, some of these steps are not used much. In such cases, they are mentioned with respective steps.
Important: Drawing correct lewis structure is important to draw resonance structures correctly.
Total Number Of Electrons Of The Valance Shells Of H2o2
There are two kind of elements in hydrogen peroxide hydrogen and oxygen. Hydrogen is a group IA element in the periodic table and only has one electron in its last shell . Oxygen is a group VIA element in the periodic table and contains six electrons in its last shell. Now, we know how many electrons are there in valence shells of hydrogen and oxygen atoms.
- valence electrons given by hydrogen atoms = 1 * 2 = 2
- valence electrons given by sulfur atom = 6*2 = 12
- Total valence electrons = 2 + 12 = 14
Don’t Miss: Algebraic Proofs Worksheets
Why Is Hydrogen Peroxide H2o2 And Not Ho
Because there is a difference between the Molecular Formula and the Empirical Formula. So to answer, H2O2 CAN be written as HO, but it is not because then the formula would not represent Hydrogen peroxide, and instead represent a hydroxyl group which has very Very different properties than a glucose molecule.
What Do Lewis Structures Show
Lewis structures, also called electron-dot structures or electron-dot diagrams, are diagrams that show the bonding between atoms of a molecule, and the lone pairs of electrons that may exist in the molecule. A Lewis structure can be drawn for any covalently-bonded molecule, as well as coordination compounds.
Recommended Reading: Beth Thomas Rad
What Does Hydrogen Peroxide Do To Hair
Hydrogen peroxide is a type of oxidative hair dye. This means it causes a chemical reaction in the hair cortex that leads to the new hair color. While oxidative dyes are more permanent than other dyes, this also means they cause oxidative stress for your hair. This stress ages your hair and can cause hair loss.
H2o Molecular Geometry Lewis Structure Shape And Bond Angles
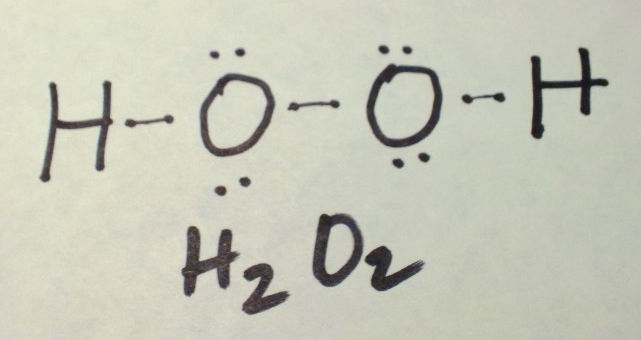
We have previously discussed the Lewis structures of CO2, O3, SO2, SO3 and more. Today we are going to learn about the Lewis structure of H2O molecule along with its molecular geometry and shape.
Water is one of the most uncomplicated chemical compounds to understand given it has a simple Lewis structure. While we always knew chemistry is everywhere, we definitely didnt know that even water has chemical formulas back in our childhood days. Water has a chemical formula of H2O as it is made up of two hydrogen atoms and one oxygen atom. This molecule also has another chemical name of Dihydrogen monoxide.
| Name of molecule | |
| No of Valence Electrons in the molecule | 8 |
| Bent |
& nbsp
In this blog, we will look at its Lewis structure, Hybridization, Molecular Geometry and Bond angles. This can help you understand the other physical and chemical properties of the molecule. But before looking at its Lewis Structure, we will first go through the total number of valence electrons for this molecule as these electrons are the ones that participate in bond formation.
Contents
Also Check: Are Elton John’s Kids His
Find The Number Of Lone Pairs Present On The Central Atom Of The H2o2 Lewis Structure
According to the H2O2 lewis structure, it contains a total of 4 lone pairs and each oxygen has 2 lone pairs.
Or you can determine lone pair in H2O2 by using the simple formula
L.P = /2
where L.P. = Lone pair on the central atom
V.E. = valence electron of that central atom
N.A. = Number of atoms attached to that central atom
So, the central atom we know Its Oxygen and It has 6 valence electrons in its last shell. Also, the number of attached atoms to each oxygen is two.
Therefore, the Lone pair on the left side oxygen is = /2 = 2 L.P.
and the lone pair on the right side of the oxygen is = /2 = 2 L.P.
So, the total number of lone pairs present on the central atom of the H2O2 lewis structure is 2 + 2 = 4.
Who Was The First Famous Peroxide Blonde
This was Jean Harlow . She was ash-blonde to begin with, but became a “platinum blonde” through regular use of hydrogen peroxide, ammonia and other chemicals, including Clorox bleach . Her film Platinum Blonde popularised the expression. She was followed by , who starred in Gentlemen Prefer Blondes and others, up to Reese Witherspoon of Legally Blonde . Not forgetting Debbie Harry, of Blondie.
Don’t Miss: Example Of Elastic Force
So What Does Hydrogen Peroxide Look Like
When it’s pure, hydrogen peroxide is an almost colourless substance that resembles water . It freezes at -0.41°C and boils at 150.2°C . Its density is 1.44 g cm-3 in the liquid state at 25°C and 1.64 g cm-3 in the solid state at -4.5°C, so that, unlike ice, solid H2O2 sinks when placed in the corresponding liquid form. It has a skewed structure with a dihedral angle of 111.5° , which minimises repulsion between the lone pairs and the O-H bond pairs. The dihedral angle is affected by hydrogen bonding it is 90.2° in solid H2O2.
What Is The Hybridization Of Ch2o
Hybridization is a theory that helps us understand the shape of molecular orbitals upon bonding to for compounds
We will not go for the deep touch of the hybridization concept, we just want to know how to find the hybridization of the CH2O molecule.
Just catch the steric number of the CH2O molecule to get it hybridized.
The steric number is simply an addition of bonded atom attached to the central atom and a lone pair present on the central atom.
Steric number of CH2O =
According to the lewis structure of CH2O, the carbon central atom is bonded with two hydrogen atoms and one oxygen atom and it contains no lone pairs.
Steric number of CH2O = = 3
| Steric number |
Don’t Miss: Quantum Physics Proves God
How To Draw Lewis Structure For Ch2o
The lewis structure of CH2O is made up of one carbon, one oxygen, and two hydrogens. These atoms are structured in a way that, the carbon atom is kept in a central position and it is connected with two hydrogens with a single bond and double-bonded with an oxygen atom.
There are a total of 2 lone pairs and 4 bonded pairs present in the lewis structure of CH2O.
Lets take a look at how to draw the lewis dot structure of CH2O with some simple steps.