How The Gas Equilibrium Constants Relate To Reaction Quotient
The process of finding the Reaction Quotient is the same as finding Kc and Kp, where the products of the reaction is divided by the reactants of the reaction \\) at any time not necessarily at equilibrium.
If a problem asks you to find which way the reaction will shift in order to achieve equilibrium, and K is given, you would have to calculate for Q and compare the two numbers.When comparing K and Q:
- K < Q : Since there are more products than reactants, the reaction will produce more reactants to reach equilibrium, the reaction favors the reactants.
- K > Q : Since there are more reactants than products, the reaction will produce more products to reach equilibrium, the reaction favors the products.
- K = Q : There is no change in the products nor reactants, so equilibrium is achieved.
A trick to remember to which what the reaction will favor is:Put:K _ Q K < Q : K \ QThe reaction will favor the reactants because reactants are on the left of the equation.K > Q : K \ QThe reaction will favor the products because products are on the right of the equation.K = Q : NO CHANGE
/Equilibria/Chemical_Equilibria/Difference_Between_K_And_Q” rel=”nofollow”> Between K and Q for more information)
Example \: Relating K to Q
Given: Kc = 1.00 at about 1100 K
CO = 1.00 mol H2O = 1.00 mol CO2 = 2.00 mol H2 = 2.00 molCompared with their initial amounts, which of the substances will be present in a greater amount and which is in a lesser amount when equilibrium is established?
Solution
Kc< Qc
Kp In Heterogeneous Equilibria
A heterogeneous equilibrium is one where reactants and products are not in same phase. For heterogeneous equilibrium the partial pressure of pure solids and liquids are not included in the equilibrium constant expression. As for example the heating of calcium carbonate in a closed vessel to form calcium oxide and carbon dioxide.
Here only carbon dioxide is in gas phase. The equilibrium constant for this reaction is
Definition Of \ And \
\ is an equilibrium constant in terms of molar concentrations and is usually defined as:
\^^}^} \nonumber \]
in the general reaction,
If a large \ is formed then there are more products formed. Inversely, a small \ indicates that the reaction favors the reactants.
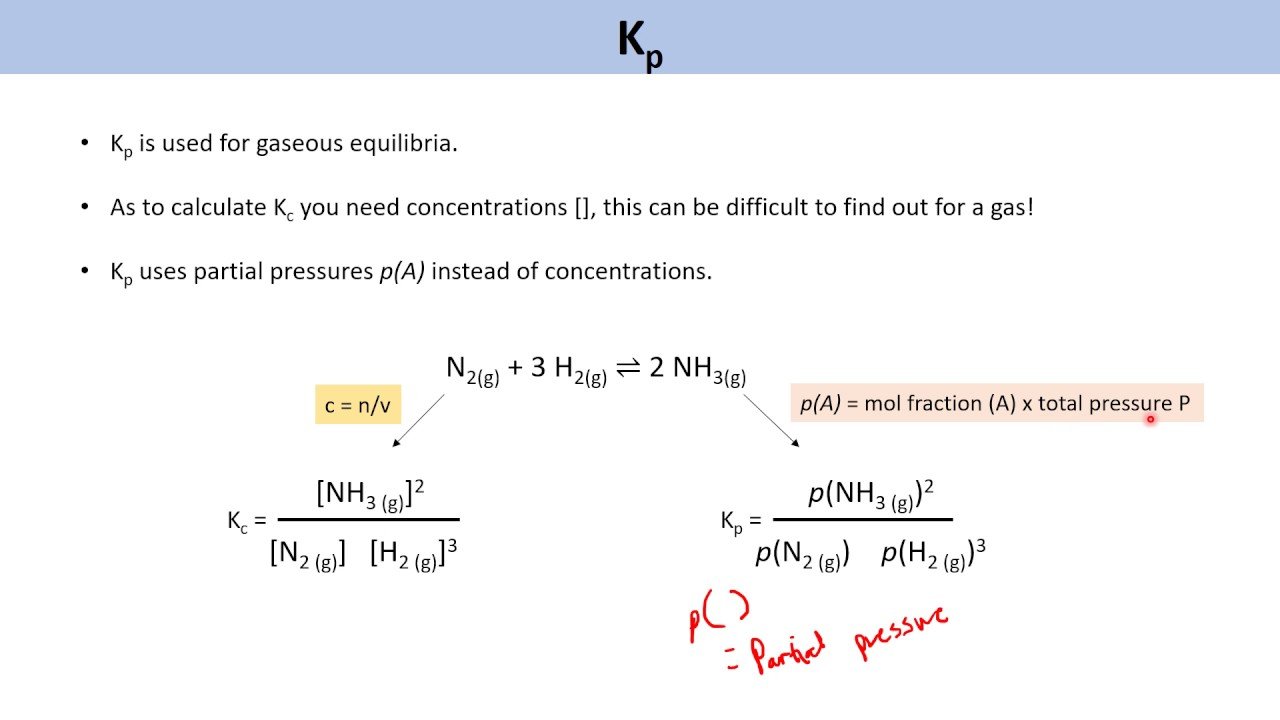
\ is an equilibrium constant in terms of partial pressures. and is usually defined as:
for the general reaction
Homogeneous Equilibria: Reactants/Products all in a single phase. For example:
Heterogeneous Equilibria: Reactants/Products in more than one phase. For example:
The relationship between the two equilibrium constants are:
where,
- \ = – . Hence \ – \nonumber \]
- \ is the gas constant found in the ideal gas law )
Also Check: Child Of Rage Beth Thomas Now
What Is Kp And How Is It Different From Kc
In some chemical reactions, the change is not permanent – the products can sometimes form the reactants. If both the forward and backward reactions occur simultaneously, then it is known as a reversible reaction. In such cases, you can calculate the equilibrium constant by using the molar concentration of the chemicals, or by using their partial pressure . In both cases, the equations are fairly similar. For the reaction:
a*A + b*B c*C + d*D,
the equilibrium constant in terms of concentration is:
Kc = / ,
where
- and are the molar concentrations of the reactants
- and are the molar concentrations of the products
The equilibrium constant formula in terms of partial pressure is:
Kp = / ,where, analogously
- Pa and Pb are partial pressures of the reactants
- Pc and Pd are partial pressures of the products
From Vapour Density Measurements:
Vapour Density and Number of Moles:
For ideal gases, pV =nRT = × RT
M = /VP = /P = /RTn = V/n = 2 × Vapour Density
Vapour density = × V/2n = × 1/n
At equilibrium V and are constant and Vapour Density is × 1/n
Vapour Density and Equilibrium:
Vapour density at start/vapour density at equilibrium = D/d = M/m = Moles at equilibrium/Moles at start.
M = initial molecular weight and m = molecular weight at equilibrium
Example:
Recommended Reading: Fsa Warm Ups Grade 4
Equilibrium Constant Reaction Quotient And Gibbs Free Energy
K is the ratio of the relative amount of products to reactants at equilibrium while Q is the ratio at any point of time of the reaction. The Q value can be compared to K to determine the direction of the reaction to take place. The spontaneity of the process is related to the free energy change. G , K , and Q are related as follows:
Equilibrium Constant vs Reaction Quotient
- Kc = Equilibrium constant measured in moles per liter.
- Kp = Equilibrium constant calculated from the partial pressures
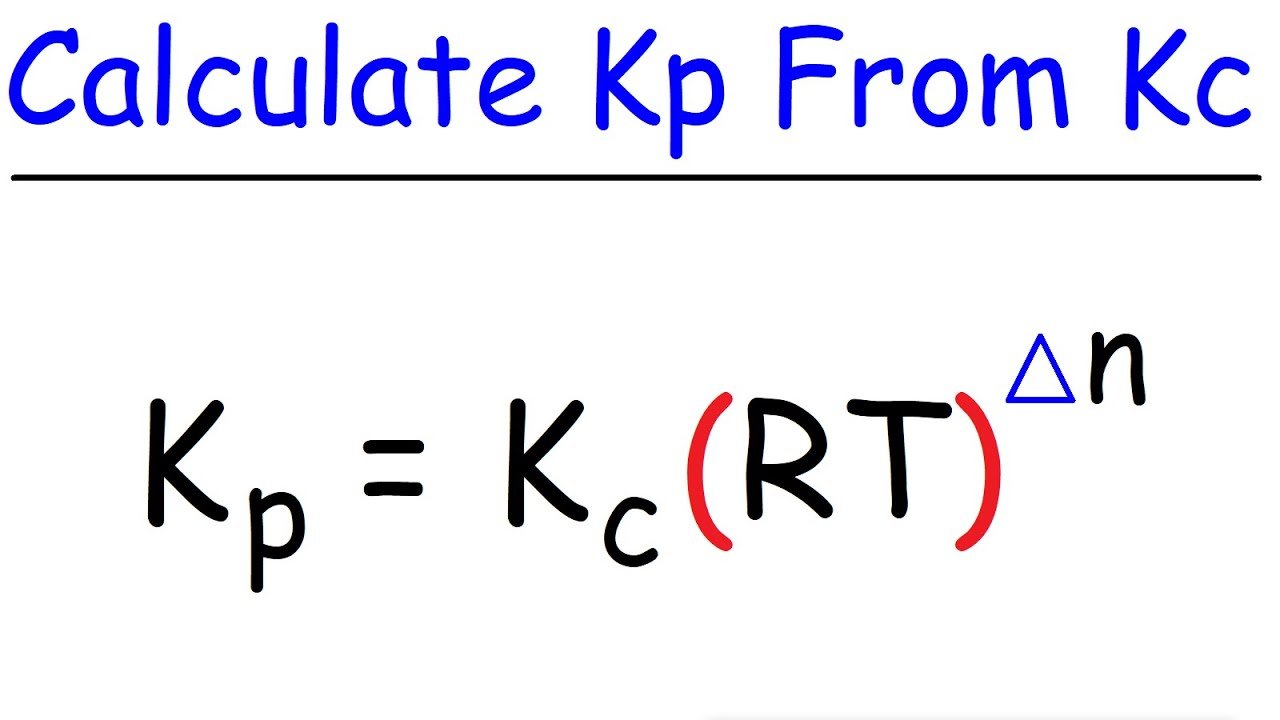
How To Calculate Kp From Kc
The relationship between Kp and Kc is:
Kp = Kc * n, where
- Kp is the equilibrium constant in terms of pressure.
- Kc is the equilibrium constant in terms of molarity.
- R is the gas constant.
- T is the temperature.
- n is the change in the number of moles:
n = mol of gaseous products – mol of gaseous reactants
To save time, start calculations with the change in the number of moles. If it’s zero then Kc equals Kp. If not, read the last paragraph of this article, use the correct units, and find the answer in less than a few minutes ).
Also Check: What Does Biotic And Abiotic Mean
Equilibrium Constant Expression In Terms Of Partial Pressures
Let us consider a general gas phase equilibrium reaction where a moles of A molecule reacts with b moles of B molecule to form c moles of C molecule and d moles of D molecule.
According to equilibrium law the equilibrium constant Kp can be written as
Here PA , PB, PC and PD are the partial pressure of gas A, B, C and D respectively. And Kp is equilibrium constant and subscript p refers to partial pressure. Partial pressures are expressed in atmosphere.
Equilibrium Constant For Predicting The Extent Of Reaction
The equilibrium constant can be used to predict the extent of a reaction, i.e. the degree of the disappearance of the reactants. The magnitude of the equilibrium constant gives an idea of the relative amount of the reactants and the products.
Case 1: The larger value of the equilibrium constant shows that forward reaction is favored i.e. the concentration of products is much larger than that of the reactants at equilibrium.
For Example:
- H2 + Br2 2HBr Kc = 5.4×1018
- H2 + Cl2 2HCl Kc = 4×1031
- H2 + 12O2 H2O Kc = 2.4×1047
This shows that at equilibrium, concentration of the products is very high , i.e. reaction go almost to completion.
Case 2: Intermediate value of equilibrium constant show that the concentration of the reactants and products are comparable.
For Example:
- Fe3 + SCN 2 Kc = 138 at 298 K
- H2 + I2 2HI Kc = 57 at 700 K.
Case 3: Low value of equilibrium constant shows that backward reaction is favored i.e. concentration of reactants is much larger than that of products i.e. the reaction proceeds to a very small extent in the forward direction.
For Example:
- N2 + O2 2NO Kc =4.8 × 10-31 at 298K
- H2O H2 + O2 Kc = 4.1 × 10-48
Also Check: Michael Jackson Kids Biological
The Significance Of The Equilibrium Constant
For any given temperature, there is only one value for the equilibrium constant. Kc only changes if the temperature at which the reaction occurs changes. You can make some predictions about the chemical reaction based on whether the equilibrium constant is large or small.
If the value for Kc is very large, then the equilibrium favors the reaction to the right, and there are more products than reactants. The reaction may be said to be “complete” or “quantitative.”
If the value for the equilibrium constant is small, then the equilibrium favors the reaction to the left, and there are more reactants than products. If the value of Kc approaches zero, the reaction may be considered not to occur.
If the values for the equilibrium constant for the forward and reverse reaction are nearly the same, then the reaction is about as likely to proceed in one direction, and the other and the amounts of reactants and products will be nearly equal. This type of reaction is considered to be reversible.
Calculating The Equilibrium Constant
For the following chemical reaction:aA + bB cC + dD
The equilibrium constant Kc is calculated using molarity and coefficients:
Kc= cd / ab
where:
, , , etc. are the molar concentrations of A, B, C, D
a, b, c, d, etc. are the coefficients in the balanced chemical equation
The equilibrium constant is a dimensionless quantity . Although the calculation is usually written for two reactants and two products, it works for any numbers of participants in the reaction.
Also Check: Value Of Kw At 25 Degrees C
Characteristics Of Equilibrium Constant
Worked Example : Calculating Equilibrium Partial Pressure

Question: A container at 800°C was filled NOCl gas which decomposes to form NO gas and chlorine gas.The equilibrium partial pressure of NOCl was 0.657 atm, what was the partial pressure of NO gas?KP = 1.8 × 10-2 for the reaction NOCl NO + ½Cl2
Solution:
Calculate the partial pressure of NO gas.P) = ? atm
Also Check: What Does Abiotic Factors Mean
Units Of Equilibrium Constant
Equilibrium constant being the ratio of the concentrations raise to the stoichiometric coefficients. Therefore, the unit of the equilibrium constant = n.
where, n = sum of stoichiometric coefficients of products sum of stoichiometric coefficients of reactants.
Also Read:
Example Equilibrium Constant Calculation
For the equilibrium between copper and silver ions:
Cu + 2Ag+ Cu2+ + 2Ag
The equilibrium constant expression is written as:
Kc = / 2
Note the solid copper and silver were omitted from the expression. Also, note the coefficient for the silver ion becomes an exponent in the equilibrium constant calculation.
You May Like: New York Integrated Algebra Textbook Answer Key
Relationship Between Kp And Kc
Assuming that all the gases in equilibrium reaction obey the ideal gas equation, the partial pressure of a gas is
PV = nRT
Divide both sides by V,
Here n/V is the molar concentration. Thus the partial pressures of individual gases A, B, C and D are:
PA = RT PB = RT PC = RT PD = RT
Substituting these value in equilibrium constant expression equation 1, we have
KP = KC –
KP = KC n ..
where n = , the difference in the sums of the coefficients for the gaseous products and reactants.
From the expression it is clear that when n=0, Kp = Kc.
What Is Equilibrium Constant
The equilibrium constant of a chemical reaction provides insight into the relationship between the products and reactants when a chemical reaction reaches equilibrium. For example, the equilibrium constant of concentration of a chemical reaction at equilibrium can be defined as the ratio of the concentration of products to the concentration of the reactants, each raised to their respective stoichiometric coefficients. It is important to note that there are several different types of equilibrium constants that provide relationships between the products and the reactants of equilibrium reactions in terms of different units.
For a chemical reaction, the equilibrium constant can be defined as the ratio between the amount of reactant and the amount of product which is used to determine chemical behaviour.
At equilibrium, Rate of the forward reaction = Rate of the backward reaction
i.e. rf = rb Or, kf × × ab = kb×× c d
At a particular temperature, the rate constants are constant. The ratio of the rate constant of forward reaction to the rate constant of backward reaction should be a constant and is called an equilibrium constant .
You May Like: Beth Thomas Cohen
Equilibrium Constant Kp Definition
When a reaction is at equilibrium, the forward and reverse reaction rate are same. The concentration of the reactants and products stay constant at equilibrium, even though the forward and backward reactions are still occurring.
When one or more of the reactants or products are gas in any equilibrium reaction, the equilibrium constant can be expressed in terms of partial pressure. Equilibrium constant expression in terms of partial pressure is designated as Kp.
Equilibrium constant Kp is equal to the partial pressure of products divided by partial pressure of reactants and the partial pressure are raised with some power which is equal to the coefficient of the substance in balanced equation.
Equilibrium Constant For Predicting The Direction Of A Reaction
The equilibrium constant can be used to predict the direction of the reaction. We need a term, reaction quotient similar to the equilibrium constant except that the conditions are not at equilibrium.
For a balanced reaction, aA + bB cC + dD
Reaction quotient is given as:
Qc = cd/ab
Qp = pcC × pdD / paA × pbB
Comparison with Kc and the direction of Reaction:
- If Q = Kc, the reaction is in equilibrium
- If Q > Kc, Q will tend to decrease so as to become equal to K. As a result, the reaction will proceed in the backward direction.
- If Q < Kc, Q will tend to increase so as to become equal to K. As a result, the reaction will proceed in the forward direction.
Recommended Reading: Angle Addition Postulate Homework 4
There’s More To Us Than What We Teach
Our award-winning faculty and small class sizes are two more reasons that KPU is a fantastic choice for your Chemistry requirements. Why spend the first year of your post-secondary education living anonymously in a class of 350? At KPU you’ll be taught in small classes by someone whose primary interest is your education.
If you have any questions about anything to do with Chemistry at KPU we’d be happy to answer them. All you have to do is send an E-mail to and we’ll do our best to give you any information you need.
Re: Hw Help Calculating Value Of Kp
Notice that reaction is the reverse of the given reaction and is also 1/2 of the given reaction. When you reverse a reaction, the new value of K becomes 1/K or K-1. Also, when you multiply an equation by some factor , then you raise K to that factor:n K=n A + n B —-n C Kn
Submitted by Ohso on Sun, 02/15/2009 – 01:38
thanks, that’s like the only thing I do understand ia that but what forumula am I using? what do I plug in for those values? because usually I am given the values for H, Br, and Hbr.
Submitted by kyle1990 on Sun, 02/15/2009 – 01:43
Also Check: Geometry Basics Segment Addition Postulate
Lecture 1: Temperature Pressure And Kp
Topics covered: Temperature, pressure and Kp
Instructor/speaker: Moungi Bawendi, Keith Nelson
ThefollowingcontentisprovidedunderaCreativeCommonslicense.YoursupportwillhelpMITOpenCourseWarecontinuetoofferhighqualityeducationalresourcesforfree.TomakeadonationorviewadditionalmaterialsfromhundredsofMITcourses,visitMITOpenCourseWareatocw.mit.edu.
PROFESSOR: I wanttoremindandclarifyasneededtheequilibriumconstantKpforgas phasereactionwastheratiosofthepartialpressuresreferencedtosomereferencepressure,whichweusuallytakeasone,onebar.Tothestoichiometry ,pDdividedbyp naught,to themu D where speciesCand Dareproducts.Andthereactantsareonthebottom.Andusuallywedon’twritep naught,butit’simportanttorememberthatit’sthere.AndthenwecanalsowritethisintermsoftheGibbsfree energyforthereaction.ThestandardGibbs freeenergy,minusdeltaG naughtofthereaction,dividedbyRT.And whatthistellsusisthatthisisanumber.Thisisanumber,there’snopre-factorherethathasunits.That’saunitlessnumber.Anditdoesn’tdependonthetotalpressure.
Sowhen welookatproblemswherewechangethepressure,thetotalpressureofthesystem,thisisgoing tostaythesame.BecauseitonlycaresaboutdeltaG naughtforthereaction.ButthisK subxwilldependonthetotal pressure. Andthat’softenasourceofconfusionin doingproblems.OK,anyquestions?We’regoing todoanexamplewherewechange thepressurefirst.
Andthatgivesyoutheapproximatevan ‘t Hoffequation.Whichisfine.Andyou’llknowthatit’sfine inproblem setsorexam,becausewe’llsayassumethatdeltaH is temperature -yes.
STUDENT: Right.
The Important Points To Be Remembered To Write The Expression Of Kp
- In equilibrium equations, even though the both sided arrows are used we consider left sided elements as reactants and right sided elements as products.
- The products are on the top in the expression .
- The reactants stays on the bottom .
- The partial pressure of the products and reactants are always raised to the power which is equal to the numerical coefficient of the respective substance in the balanced chemical equation.
- For any heterogeneous system the partial pressure of pure solids and liquids are not included in the equilibrium constant expression.
Recommended Reading: Beth Thomas Age
Need A Science Course But You’re Not A Science Student
Ever wonder how you could use super glue to take fingerprints or how blood alcohol content is really measured? Earn credits while you learn the answers to these questions and others Chemistry 1101, our Chemistry course for non-science majors, will give you a hands-on insight into the world of forensic science. Don’t worry: we’ve designed Chemistry 1101 specifically for the student who does not have any background in chemistry. As a result, this course has something to offer everyone, from the aspiring teacher who’ll appreciate the real-world examples to the curious general arts student who just wants to find out more about forensic science.
If you’re a science or engineering student, you should know that Chemistry 1101 cannot be used as credit towards your program completion.