Given The Equation: 2h2 + O2 2h2o If Given 10g H2 Gas And 15g O2 Gas What Is The Limiting Reactant
Start by taking a look at the balanced chemical equation for this reaction
#color”H”_text + “O”_text -> 2″H”_2″O”_text#
Notice that you have a #color:1# mole ratio between hydrogen gas and oxygen gas.
This means that, regardless of how many moles of oxygen gas you have, the reaction needs twice as many moles of hydrogen gas in order to proceed.
You know that you start with #”10.0 g”# of hydrogen gas nad #”15.0 g”# of oxygen. To determine how many moles of each you have, use their respective molar masses
#10.0color)) * /))) = “4.961 moles H”_2#
#15.0color)) * /))) = “0.4688 moles O”_2#
Notice that you have quite a significant difference, way bigger than the required #color:1# ratio needed, between how many moles of hydrogen gas and how many moles of oxygen you have.
This means that you’re dealing with a limiting reagent. How many moles of oxygen would have been needed to react with all the hydrogen?
#4.961color)) * /color))) = “2.4805 moles O”_2#
Since you don’t have that many moles of oxygen, it follows that oxygen is your limiting reagent, i.e. it will determine how much hydrogen reacts and how much remains in excess.
For example, how many moles of hydrogen gas would react if you have #0.4688#
#0.4688color)) * ” moles H”_2)/))) = “0.9376 moles H”_2#
The rest of the hydrogen will be in excess.
What Are Some Real Life Examples Of Combustion Reactions
Combustion Reaction Examples
- Burning wood. Burning wood in a fire is an example of a combustion reaction.
- A Lighted match. A lighted match is another example of a combustion reaction.
- Burning coal. Burning coal qualifies as a combustion reaction because coal transforms from a solid element to a vapor during the process.
- Fireworks.
Why Do I Feel Connected To Water
Were naturally drawn to aquatic hues and people associate this color with qualities like calm, openness, depth and wisdom. We are beginning to learn that our brains are hardwired to react positively to water and that being near it can calm and connect us, increase innovation and insight, and even heal whats broken.
Don’t Miss: Is Paris Jackson Michael Jackson’s Biological Daughter
More Information Onmolar Mass And Molecular Weight
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom in the formula by the formula weight and multiplying by 100.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Finding molar mass starts with units of grams per mole . When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
What Kind Of Chemical Reaction Is Ch4 O2 Co2 H2o
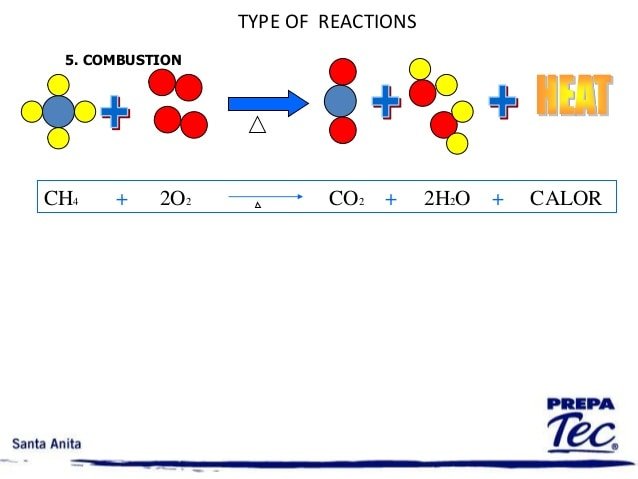
Keeping this in view, what type of reaction is ch4 2o2 co2 2h2o?
Incomplete combustion
Subsequently, question is, is ch4 2o2 co2 h2o a redox reaction? CH 4 is a reducing agent, O 2 is an oxidizing agent. , . Colorless, odorless gas. [Note: Shipped as a liquefied compressed gas. Search by reactants and by products
| 1 2 | |
|---|---|
| O2 + CH4 + C3H8 H2O + CO2 | |
| 10 | O2 + CH4 H2O + CO2 + C2H2 |
| 1 2 |
In this regard, what is the balanced equation for ch4 o2 co2 h2o?
Chemical Equation Balancer CH4 + O2 = CO2 + H2O.
What type of reaction is carbon dioxide and water?
Simple answer is Carbon dioxide reacts with water to form Carbonic acid which is weak and dissociates partly into bicarbonate ions. In terms of observable changes, the water gets weakly acidic which although can be detected on a pH scale.
You May Like: Does Kamala Harris Have Any Biological Children
Effect On Biological Systems
Different isotopes of chemical elements have slightly different chemical behaviors, but for most elements the differences are far too small to have a biological effect. In the case of hydrogen, larger differences in chemical properties among protium , deuterium, and tritium occur, because chemical bond energy depends on the reduced mass of the nucleuselectron system this is altered in heavy-hydrogen compounds more than for heavy-isotope substitution involving other chemical elements. The isotope effects are especially relevant in biological systems, which are very sensitive to even the smaller changes, due to isotopically influenced properties of water when it acts as a solvent.
Heavy water affects the period of circadian oscillations, consistently increasing the length of each cycle. The effect has been demonstrated in unicellular organisms, green plants, isopods, insects, birds, mice, and hamsters. The mechanism is unknown.
To perform their tasks, enzymes rely on their finely tuned networks of hydrogen bonds, both in the active center with their substrates, and outside the active center, to stabilize their tertiary structures. As a hydrogen bond with deuterium is slightly stronger than one involving ordinary hydrogen, in a highly deuterated environment, some normal reactions in cells are disrupted.
Other Heavy Forms Of Water
Semiheavy water, HDO, exists whenever there is water with light hydrogen and deuterium in the mix. This is because hydrogen atoms are rapidly exchanged between water molecules. Water containing 50% H and 50% D in its hydrogen actually contains about 50% HDO and 25% each of H2O and D2O, in dynamic equilibrium.In normal water, about 1 molecule in 3,200 is HDO , and heavy water molecules only occur in a proportion of about 1 molecule in 41 million . Thus semiheavy water molecules are far more common than “pure” heavy water molecules.
O is also commercially available, e.g., for use as a non-radioactive isotopic tracer. It is “heavy water” as it is denser than normal water but is rarely called heavy water, since it does not contain the deuterium that gives D2O its unusual nuclear and biological properties. It is more expensive than D2O due to the more difficult separation of 17O and 18O. H218O is also used for production of fluorine-18 for radiopharmaceuticals and radiotracers and for positron emission tomography. Small amounts of 17O and 18O are naturally present in water and most processes enriching heavy water also enrich the heavier isotopes of oxygen as a side-effect. This is undesirable if the heavy water is to be used as a neutron moderator in nuclear reactors, as 17O can undergo neutron capture followed by emission of an alpha particle producing radioactive 14C
Recommended Reading: What Does Abiotic Mean In Biology
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
What Is The Chemical Name Of Gypsum
Its chemical name is calcium sulfate dihydrate .
Gypsum can be mixed with water and restored to its original rock-like condition. This means it can be more rigid. It has a closed recycling loop that can be reused indefinitely while maintaining high quality.
An alternative to natural gypsum is flue gas desulfurization gypsum or FGD gypsum. It is a by-product of coal-fired power plants.
What are the uses of gypsum?
Gypsum is used for the renovation and construction of all types of residential and non-residential buildings. It can be used for very beautiful, contemporary designs and structures, as well as simple everyday objects and processes.
European building interiors use about 5 million tones of gypsum-based plaster every year. European interior surfaces over 1,500 million square meters
Gypsum uses include: wallboard, cement, plaster of Paris, soil conditioning, and hardening of Portland cement. The varieties of gypsum are known as satin spar and alabaster for a variety of ornamental purposes however, their low hardness limits their durability.
Some examples of gypsum:
Plaster board
Plasterboard is used for the partitioning and lining of walls, ceilings, ceilings and floors. Gypsum is an important component of modern plasterboard.
Gypsum fiberboard
Gypsum fiberboard is used for partitions, walls, ceilings, ceilings and floors. Standard gypsum fiberboard is soundproof, as well as good for impact and moisture resistance.
Building plaster
Plaster block
Read Also: Holt Geometry Lesson 4.5 Practice B Answers
What Type Of Reaction Is Ch4 2o2 Co2 2h2o
4.6/5
Herein, what type of reaction is ch4 2o2 — co2 2h2o?
Types of chemical reactions
| What type of reaction is: CH4 + 2O2 ———-CO2 + 2H2O | |
| electrolysis | What type of reaction is: 2NaCl ———- 2Na + Cl2 |
| dissolving | What type of reaction is: C12H22O11 ———- C12H22O11 |
One may also ask, is ch4 2o2 co2 2h2o a combustion reaction? In this demonstration methane gas or natural gas is the hydrocarbon that reacts with oxygen as shown below: CH4 + 2O2 CO2 + 2H2O There are two types of combustion reactions: complete and incomplete combustion. Therefore there are four products, carbon dioxide, water, carbon, and carbon monoxide.
In this manner, what type of reaction is ch4 2o2?
CH4 + 2O2 CO2 + 2H2O Incomplete combustion carbon monoxide and water.
Is ch4 2o2 co2 2h2o endothermic or exothermic?
It is not an endothermic reaction. Combustion always involves the addition of O2,in combustion always energy is released and this energy is used to break the bonds of reactants. So combustion of methane is an exothermic reaction. CH4 +O2 gives CO2+H2O,H=-21 KCal.
Heavy Water Radiation Contamination Confusion
Although many people associate heavy water primarily with its use in nuclear reactors, pure heavy water is not radioactive. Commercial-grade heavy water is slightly radioactive due to the presence of minute traces of natural tritium, but the same is true of ordinary water. Heavy water that has been used as a coolant in nuclear power plants contains substantially more tritium as a result of neutron bombardment of the deuterium in the heavy water .
In 1990, a disgruntled employee at the Point Lepreau Nuclear Generating Station in Canada obtained a sample of heavy water from the primary heat transport loop of the nuclear reactor, and loaded it into a cafeteria drink dispenser. Eight employees drank some of the contaminated water. The incident was discovered when employees began leaving bioassay urine samples with elevated tritium levels. The quantity of heavy water involved was far below levels that could induce heavy water toxicity, but several employees received elevated radiation doses from tritium and neutron-activated chemicals in the water. This was not an incident of heavy water poisoning, but rather radiation poisoning from other isotopes in the heavy water.
Don’t Miss: Beth Thomas Parents
Metabolic Rate Testing In Physiology And Biology
Tritium is the active substance in self-powered lighting and controlled nuclear fusion, its other uses including autoradiography and radioactive labeling. It is also used in nuclear weapon design for boosted fission weapons and initiators. Tritium undergoes beta decay into Helium-3, which is a stable but rare isotope of Helium that is itself highly sought after. Some tritium is created in heavy water moderated reactors when deuterium captures a neutron. This reaction has a small cross-section and produces only small amounts of tritium, although enough to justify cleaning tritium from the moderator every few years to reduce the environmental risk of tritium escape.
Producing a lot of tritium in this way would require reactors with very high neutron fluxes, or with a very high proportion of heavy water to nuclear fuel and very low neutron absorption by other reactor material. The tritium would then have to be recovered by isotope separation from a much larger quantity of deuterium, unlike production from lithium-6 , where only chemical separation is needed.
How Do You Balance H2o2 H2o O2
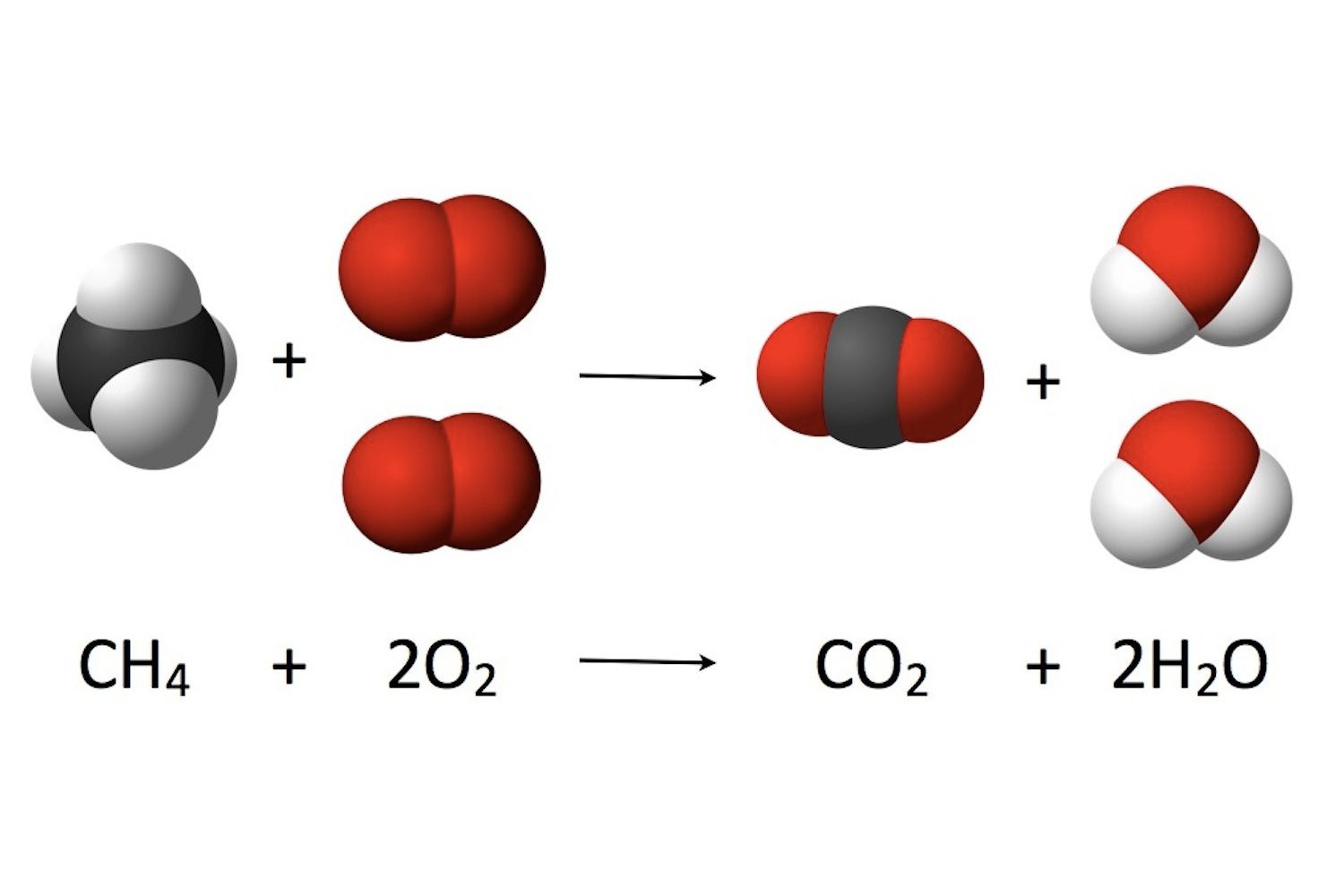
Explanation: This reaction is of the spontaneous decomposition of hydrogen peroxide down into water and oxygen. Add 2 molecules of hydrogen peroxide and 2 molecules of water. Since oxygen is naturally diatomic, the total number of atoms of each element is now the same on both sides of the equation so it is balanced.
Don’t Miss: Holt Geometry Lesson 4.5 Practice B Answers
How Do You Balance A Chemical Equation
In order to balance the chemical equation, you need to make sure the number of atoms of each element on the reactant side is equal to the number of atoms of each element on the product side. In order make both sides equal, you will need to multiply the number of atoms in each element until both sides are equal.
How Do You Bless Water Spiritually
Once youve made your holy water, you can bless it using the following prayer from the Novus Ordo Book of Blessings: Blessed are you, Lord, Almighty God, who deigned to bless us in Christ, the living water of our salvation, and to reform us interiorly, grant that we who are fortified by the sprinkling of or use of
Don’t Miss: Theory Of Everything Geometry Dash Song
What Is The Substrate In 2h2o2 2h2o O2
21 Cards in this Set2H2O2-> > > > 2H2O+O2 name the reactants in this equationhydrogen peroxidepresented with two test tubes:One of the tubes has more catalase than the other, if the same amount of hydrogen peroxide is simultaneously added to both predict what will happen?one will have a bigger reaction19 more rows
Why Does Combining Hydrogen And Oxygen Typically Produce Water Rather Than Hydrogen Peroxide
Chemists Joel Rosenthal and Daniel G. Nocera of the Massachusetts Institute of Technology provide this answer.
When molecular hydrogen and oxygen are combined and allowed to react together, energy is released and the molecules of hydrogen and oxygen can combine to form either water or hydrogen peroxide. These two processes are represented by the two chemical equations shown at right. Chemists use redox half-reactions to describe thermodynamic processes like the ones embodied by such equations. For both of the reactions shown, the hydrogen molecules are oxidized and the oxygen atoms are reduced. Accordingly, each of the reactions below is described by a combination of two half-reactions–one corresponding to a chemical oxidation and another corresponding to a reduction.
Recommended Reading: Does Mj Have Any Biological Kids
What Are The Hazards Of Silver Nitrate
Exposure to Silver Nitrate can cause headache, dizziness, nausea and vomiting. to transport Oxygen, causing headache, fatigue, dizziness, and a blue color to the skin and lips . Exposure to very high levels can cause trouble breathing, collapse and even death.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Also Check: What Is Figure Ground In Psychology
What Is The Chemical Equation For Water
H2O2H2 + O2 2H2O Chemical equations give the following information about chemical reactions. Chemical equations show the formulas for the substances that take part in the reaction. The formulas on the left side of the arrow represent the reactants, the substances that change in the reaction.
What is the chemical equation for H2O?, For example, it is known that two molecules of hydrogen gas, H2, react with one molecule of oxygen gas, O2, to form two molecules of water, H2O. This reaction may be represented by the chemical equation 2H2+O22H2O.
Furthermore, What type of reaction is H2 O2 H2O?, Type of Chemical Reaction: For this reaction we have a Combination reaction. Balancing Strategies: For this reaction it is helpful to start by changing the coefficient in front of H2O and so that you have an even number of oxygen atoms.
Finally, What type of reaction is H2 O2 h2o?, Type of Chemical Reaction: For this reaction we have a Combination reaction. Balancing Strategies: For this reaction it is helpful to start by changing the coefficient in front of H2O and so that you have an even number of oxygen atoms.