How Are Ph And Poh Related To Kw
We use Kw to find the pH and pOH because Kw has a set value of 1.0 x 10^-14 which can be plugged into this equation to find the values of pH and pOH. In addition, -logKw = 14 which is then just plugged into the equation and used to determine either pH or pOH depending on which concentration you have found.
Understanding Kb And Pkb
Kb is the base dissociation constant. The base dissociation constant is a measure of how completely a base dissociates into its component ions in water.
- Kb = /
- pKb = -log Kb
A large Kb value indicates the high level of dissociation of a strong base. A lower pKb value indicates a stronger base.
pKa and pKb are related by the simple relation:
- pKa + pKb = 14
A Closer Look At Autoionization Constant Kw
Autoionization constant is expressed as demonstrated in equation below.
Kw =
At this point, it is prudent to appreciate that when writing Kw constant chemistry expressions, you omit the concentrations. What does this imply when writing or working on Kw? It means that Kw, in this case, is a pure water sample.
Note that Kw is dependent on temperature. For example, you can calculate the Kw value at 250C utilizing H3O+, which is closely related to water PH. At 25 degrees, the PH of pure water is 7. Therefore, what is the concentration of hydronium ions in such a sample? Here is a demonstration in equation :
At 250C =10pH=107 M
From chemistry Kw, we know that the concentration of hydroxide and hydronium ions follows the ration of 1:1 in the autoionization of water equation. Therefore, we can advance equation above to calculate hydroxide ions, as demonstrated in equation below:
At 250C ==107 M
In many cases, students find it hard to visualize the entire reaction. However, 10-7 is a very small figure, and, therefore, only a relatively small quantity of water will be in the ionized form. From equation above, we can go ahead and calculate Kw of water at 250C. See the equation of how to calculate Kw chemistry below:
Kw=×=1014 at 25C
Read Also: Exponential Modeling With Percent Growth And Decay Common Core Algebra 2 Homework
Understanding Ka And Pka
Ka, pKa, Kb, and pKb are most helpful when predicting whether a species will donate or accept protons at a specific pH value. They describe the degree of ionization of an acid or base and are true indicators of acid or base strength because adding water to a solution will not change the equilibrium constant. Ka and pKa relate to acids, while Kb and pKb deal with bases. Like pH and pOH, these values also account for hydrogen ion or proton concentration or hydroxide ion concentration .
Ka and Kb are related to each other through the ion constant for water, Kw:
- Kw = Ka x Kb
Ka is the acid dissociation constant. pKa is simply the -log of this constant. Similarly, Kb is the base dissociation constant, while pKb is the -log of the constant. The acid and base dissociation constants are usually expressed in terms of moles per liter . Acids and bases dissociate according to general equations:
- HA + H2O â A- + H3O+
In the formulas, A stands for acid and B for base.
- Ka = /
- pKa = – log Ka
- at half the equivalence point, pH = pKa = -log Ka
A large Ka value indicates a strong acid because it means the acid is largely dissociated into its ions. A large Ka value also means the formation of products in the reaction is favored. A small Ka value means little of the acid dissociates, so you have a weak acid. The Ka value for most weak acids ranges from 10-2 to 10-14.
What Does Kw Mean In Chemistry
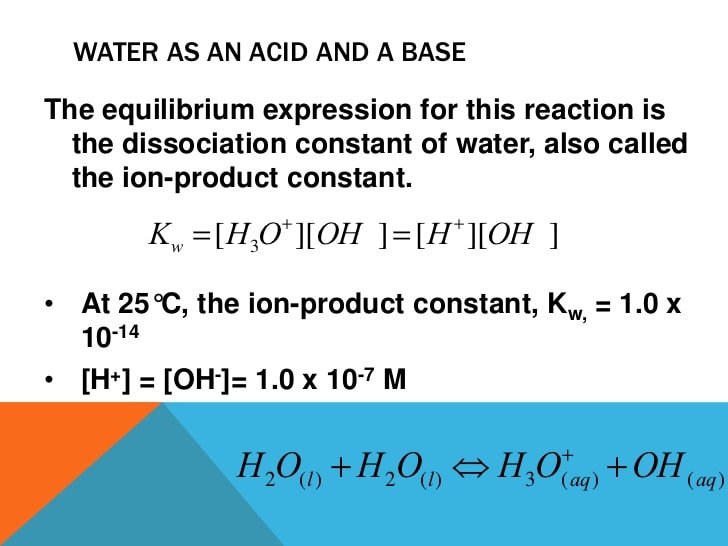
Water contains both acidic and basic molecules. Because acids and bases will always react when put together, it simply means that water will react with itself! This sounds very strange. But in reality, it actually happens. The water molecules exchange protons in a process referred to as autoionization of water. This process can be expressed in the following equation below:
H2O+H2OH3O++OH
In the above equation, one water molecule can be seen donating a proton and, therefore, acts as a Bronsted-Lowry acid. Then, another molecule accepts the molecule and, therefore, acts as a Bronsted-Lowry base. After the reaction, two molecules are formed, Hydronium ions and hydroxide ions. This reaction takes place all the time in any quantity of water.
If you have a sample of pure water, it means that the concentration of Hydronium ions and hydroxide ions is equal. Here is a demonstration in an equation .
In pure water: =
It is important to note that the process demonstrated in the equation above is easily reversible because water is a weak base and a weak acid. To establish the concentrations, it is important to look at the next concept of the autoionization constant.
You May Like: Is Paris Jackson Michael’s Biological Daughter
The Effective Range Of The Ph Scale
It is common that the pH scale is argued to range from 0-14 or perhaps 1-14 but neither is correct. The pH range does not have an upper nor lower bound, since as defined above, the pH is an indication of concentration of H+. For example, at a pH of zero the hydronium ion concentration is one molar, while at pH 14 the hydroxide ion concentration is one molar. Typically the concentrations of H+ in water in most solutions fall between a range of 1 M and 10-14 M . Hence a range of 0 to 14 provides sensible “bookends” for the scale ). One can go somewhat below zero and somewhat above 14 in water, because the concentrations of hydronium ions or hydroxide ions can exceed one molar. Figure 1 depicts the pH scale with common solutions and where they are on the scale.
Figure \: Solutions and the placement of them on pH scale
- From the range 7-14, a solution is basic. The pOH should be looked in the perspective of OH- instead lf H+. Whenever the value of pOH is greater than 7, then it is considered basic. And therefore there are more OH- than H+ in the solution
- At pH 7, the substance or solution is at neutral and means that the concentration of H+ and OH- ion is the same.
- From the range 1-7, a solution is acidic. So, whenever the value of a pH is less than 7, it is considered acidic. There are more H+ than OH- in an acidic solution.
Note
The pH scale does not have an upper nor lower bound. Negative pH values are possible
Example \
SOLUTION
| Pancreas Secretions | 8.1 |
Meaning Of Kw The Ionic Product For Water
What is the + for water?
Kw= = 1.0x 10 ^-14
this was an example in chem class but i didnt understnd it even asking the teacher…what does Kw stand for…the brackets…and whats the problem really asking??? plz help!!!
First of all the brackets mean “the concentration of ….” whatever is in the brackets. For instance, means the concentration of H+ ion .
Kw is the ionization constant for water. It is a type of equilibrium constant referring to the equilibrium that exists any time water is present: H2O H+ + OH-The law of mass action says that there is an equilibrium constant for this equilibrium reaction and it would have the form. Keq = / However, the value for the for liquid water is a constant and can be moved to the same side as the Keq. Keq = Since the is a constant, and Keq is a constant, they can be combined together to give a new constant Kw = Kw has been measured many times and at 25C has been found to have a value of 1.0 x 10-14
This is a very important equation, because any time we know the value of the we can find the value for and vice versa.
I have never seen the expression + = ? I would think that it could have an infinite number of answers depending on what the is.
This is an extremely useful equation and my quess is that was what you were being asked about.
thank u so much! u really helped clear that up for me
yea…it was asking about the ph and poh
Also Check: Which Of The Following Perspectives Dominated American Psychology For Decades
What Is The Relationship Between Ph And H+
Hydrogen ion concentration is more conveniently expressed as pH, which is the logarithm of the reciprocal of the hydrogen ion concentration in gram moles per liter. Thus, in a neutral solution the hydrogen ion and the hydroxyl ion concentrations are equal, and each is equal to 107. A pH of 7 is neutral.
Ionic Product Of Water Kw
Water can act as an acid or a base. And the following equilibrium exists in water:
This can be expressed as an equilibrium constant using the concentrations. However, the equilibrium lies very far to the left. This means that very few hydrogen and hydroxide ions are produced and the concentration of water can be taken to be constant. Therefore we get a different expression that is called Kw.
Now that we have this information it is possible to calculate the pH of strong bases, by a very simple process have a look at the following example.
Read Also: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
What Is Kw Defined As
kW stands for kilowatt. A kilowatt is simply 1,000 watts, which is a measure of power. So a 1,000 watt drill needs 1,000 watts of power to make it work, and uses 1 kWh of energy in an hour. Thats why, if you leave a TV or computer on standby, it is still using power and creating a kWh cost on your energy bill.
Does Adding H+ Change Ph Solution
Adding water to an acid or base will change its pH. Water is mostly water molecules so adding water to an acid or base reduces the concentration of ions in the solution. When an acidic solution is diluted with water the concentration of H + ions decreases and the pH of the solution increases towards 7.
Recommended Reading: What Does Abiotic
Kw Increases With Increase Of Temperature
Autoionisation of water is an endothermic process. According to Le chateliers principle, if conditions are changed in a equilibrium process, the equilibrium will shift to such a direction where it can minimize the effect of the change of the condition. Thus if water is heated the equilibrium will shift to right to form more ions by absorbing extra heat as this is an endothermic process. According to the equation of Kw, if the concentration of ions increases the Kw increases. So we can say that Kw increases with the increase of temperature.
Kw Of Water And Temperature Changes
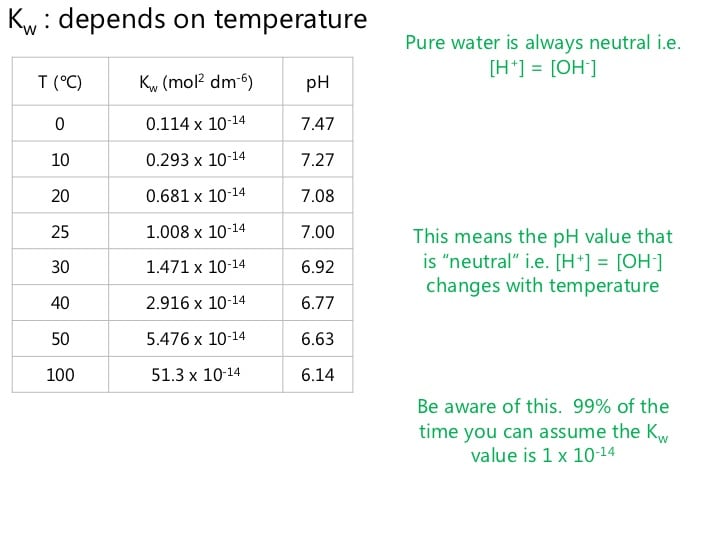
One thing you need to appreciate when looking at Kw definition chemistry and Kw constant is that the PH varies depending on temperatures. Using the equation , the first equation in this post, a forward reaction absorbs heat.
H2O+H2OH3O++OH
Using Le Chateliers Principle, you will find that raising the temperature of water results in the equilibrium working to lower the temperature. This is achieved through heat absorption. It also implies that forward reaction is going to be favored The overall impact will be lowering of Kw as the temperature moves up. To demonstrate the changes to Kw as temperature changes, have a look at the table below:
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
What Can A 1 Kw Generator Power
1 kW to 10 kW generators Homes and small offices can work on 1 kW to 10kW generators. A 5kW generator can power up to four lights, a fan, an electric motor and a refrigerator. Small homes can function with such a basic generator. However, the ideal generator for a home should at least be of 10 kW capacity.
Key Concepts And Summary
The concentration of hydronium ion in a solution of an acid in water is greater than 1.0 × 107M at 25 °C. The concentration of hydroxide ion in a solution of a base in water is greater than 1.0 × 107M at 25 °C. The concentration of H3O+ in a solution can be expressed as the pH of the solution pH = logH3O+. The concentration of OH can be expressed as the pOH of the solution: pOH = log. In pure water, pH = 7.00 and pOH = 7.00
Read Also: Algebra 2 Eoc Fsa Practice Test
Introduction To The Water Ionization Constant Kw
Pure water undergoes auto-ionization or self-ionization by donating or accepting a proton between two molecules of water to form H3O+ and OH ions. This is also known as autoprotolysis or amphoteric nature of water.
The hydronium ion is a very strong acid and hydroxide ion is a very strong base. Thus they can associate again to form water molecule. So water molecules and the ions always stay in equilibrium. And the equilibrium lies to the left. Thus a very small amount of hydronium ions and hydroxide ions are found in water.
The equilibrium constant for this autoionisation of water is known as Kw. Thus
Kw =
Or simply Kw = .
Here we omit the concentration of water molecule which should stay as a denominator. The reason is, not much change in concentration is observed during this process.
Acidic Basic And Neutral Solution
In pure water the concentration of hydronium ion and hydroxide ion are same and equal to 10-7 M at 250 C. This types of solution is known as neutral solution. But depending on the difference between their concentration, the solution is named as acidic or basic. Such as
- If = , it is a neutral solution.
- If > , it is an acidic solution.
- If < , it is a basic solution.
Recommended Reading: How Many Subfields Of Psychology Are There
What Is The Relationship Between Kw Ka And Kb
Ka, Kb and Kw are related in a simple equation: Ka multiplied by Kb equals Kw. This equation can be used to determine any of the variables if the other two variables are known.
Ka, or the acid dissociation constant, is an equilibrium constant for the dissociation of acids. Strong acids completely dissociate in water, while weak acids only partially dissociate. The Ka of an acid shows the strength or weakness of an acid. Strong acids have large Ka values because they completely dissociate in water, and weak acids have small Ka values. Kb, or the base dissociation constant, is the equilibrium expression for bases. In water strong and weak bases both establish an equilibrium value. This value is denoted by the Kb value. Kw is the water dissociation constant and is defined as the dissociation and ionization of water. Kw is always 1.0 x 10^-14 when the water is at 25 degrees Celsius. The relationship between Kw, Kb and Ka allows chemistry students to determine the relative strength of an acid or a base by comparing it to the Kw value. For example, if the Ka value of an acid is known, then the Kb value of the acids conjugate base can be found by plugging in the Ka and Kw values into the previously mentioned equation.
Does The Value Of Kw Remain Constant When The Solution Becomes Acidic Or Basic Due To Hydrolysis Of Salt
When few drops of acid or is added to water Kw remains constant does the same happens in case hydrolysis of salts. I want to understand the topic completely and to know about the concepts in detail
Kw represents the dissociation of water at normal condition and at 25ºC. This value is constant and is not affected whether the solution is acidic or basic. Its value is equal to 10-14. Maybe you are talking about Ka and Kb. These values represent the acid and base dissociation constants respectively which means how a certain acid is dissociated under specific conditions.
Recommended Reading: Algebra 1 Age Word Problems
What Does The P Mean
Whenever you see a “p” in front of a value, like pH, pKa, and pKb, it means you’re dealing with a -log of the value following the “p”. For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. pH is the -log of hydrogen ion concentration, and so on.