What Is Sulfur Dioxide
Sulfur Dioxide , also known as Sulfur Dioxide , is the entity of a bond between sulfur and oxygen atoms. It is a colorless, toxic, inorganic gas with a pungent smell like nitric acid. It is naturally released by volcanic activity.It gives a weak acid solution when dissolved in water. Sulfur dioxide is naturally found in small amounts in the atmosphere and is a primary precursor of sulfuric acid.
S For Drawing The Lewis Dot Structure Of So2
1. Count the total valence electrons in SO2
The Lewis dot structure of a molecule is a simplified representation of all the valence electrons present in the molecule. Therefore, the first step whenever we want to draw the Lewis structure of a molecule is to calculate the total valence electrons present in it.
Valence electrons present in an atomic element can be easily determined by using the Periodic Table. Having a quick look at the Periodic Table, we can readily identify that both Sulfur and oxygen are situated in Group VI A. So, both atoms have 6 valence electrons each.
How To Draw Lewis Structure Of So2
The Lewis structure of SO2 consists of a sulfur atom present at the center of the molecule. It is bonded with the help of two double bonds to two atoms of oxygen at the sides. There are a total of 5 lone pairs in the SO2 lewis structure.
Lets draw the Lewis structure of SO2 using the following simple guidelines.
Recommended Reading: Geometry Dash Blast Processing Full Ver
So2 Is Polar Or Nonpolar
Sulfur dioxide is polar in nature.The difference in electronegativity between sulfur and oxygen atoms creates polarity in the molecule.Oxygen has a greater electronegative potential than sulfur.Therefore, oxygen exerts more pull on the covalent bonds in sulfur dioxide.The portion of the molecule that has both oxygen atoms on it is slightly negatively charged.whereas the portion that has the sulfur atom has a slightly positive charge.This makes SO2 a polar molecule like H2S.In addition, the unbonded electrons on the sulfur and oxygen create repulsion between atoms.This is another cause of the polarity of the sulfur dioxide molecule.Check the full article SO2 is polar or nonpolar?.
Chapter : Predicting The Geometry Of Molecules
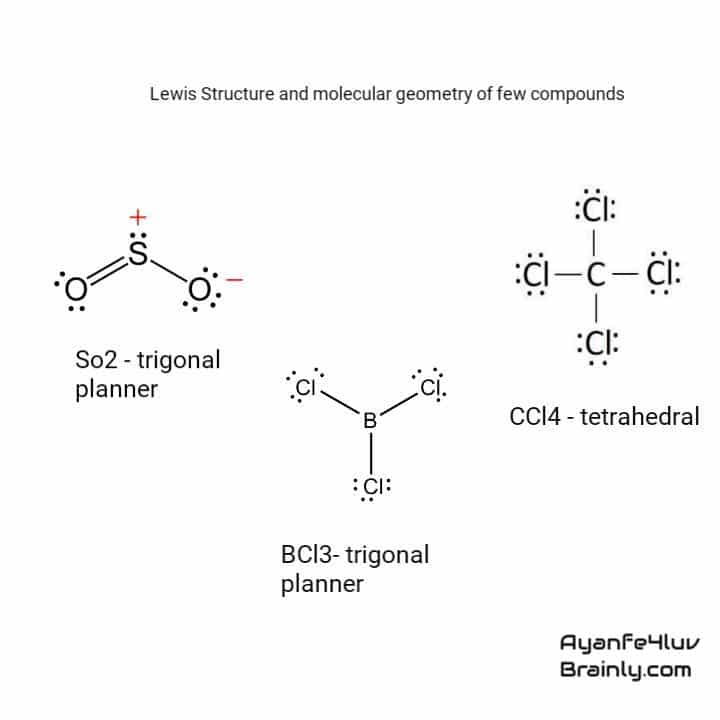
General Chemistry for EngineeringCHM 2000
- To use the VSEPR model to predict molecular geometries.
- To predict whether a molecule has a dipole moment.
We continue our discussion of structure and bonding by introducing the valence-shell electron-pair repulsion model A model used to predict the shapes of many molecules and polyatomic ions, based on the idea that the lowest-energy arrangement for a compound is the one in which its electron pairs are as far apart as possible. , which can be used to predict the shapes of many molecules and polyatomic ions. Keep in mind, however, that the VSEPR model, like any model, is a limited representation of reality the model provides no information about bond lengths or the presence of multiple bonds. Following sections of this chapter will connect the VSEPR model to atomic and molecular orbitals.
You May Like: What Is Migration In Human Geography
S02 Is Polar Or Nonpolar
Sulfur dioxide is polar in nature. The difference in electronegativity between sulfur and oxygen atoms creates polarity in the molecule. Oxygen has a greater electronegative potential than sulfur. Therefore, oxygen exerts more pull on the covalent bonds in sulfur dioxide. The portion of the molecule that has both oxygen atoms on it is slightly negatively charged.
Whereas, the portion which has the sulfur atom has a slightly positive charge. This makes SO2 a polar molecule like H2S. In addition, the unbonded electrons on the sulfur and oxygen create repulsion between atoms.
This is another cause of the polarity of the sulfur dioxide molecule.
What Is Sulfur Hexafluoride
Sulfur hexafluoride is a non-toxic gas that is used in a variety of applications due to its inert properties. While SF6 is non-toxic when used properly, toxic byproducts can be produced during electrical discharges within SF6-filled equipment, posing a threat to the health of workers who come into contact with them.
Recommended Reading: How To Determine Melting Point Chemistry
Molecular Geometry Of So2
The SO2 molecule has an asymmetric bent or V-shape. The sulfur atom lies at the center of the inverted V shape while the oxygen atoms occupy the terminals. The presence of a lone pair of electrons on the central sulfur atom makes sure that the O atoms are maximally pushed away from the center.
The lone pair-bond pair repulsions are significantly stronger than the bond pair-bond pair repulsions so the O-atoms tilt slightly closer to each other but far away from the central S-atom in an angular shape.
It should be noted that the presence of a lone pair has a pronounced effect on the molecular geometry or shape of a molecule. On the other hand, the electron geometry depends on the total number of electron pairs present on the central atom in the molecule.
Therefore, we seriously take into consideration the repulsive effect of the lone pair on the central S atom in the SO2 molecule when determining its molecular geometry. But this repulsive effect can be ignored while considering the ideal electronic geometry of SO2.
Is So2 Polar Or Nonpolar
A specific electronegativity difference of 0.86 units exists between the bonded sulfur and oxygen atoms in a S=O bond.
Paulings electronegativity scale states that a polar bond has an electronegativity difference greater than 0.5 units between its bonded atoms.
As 0.86 > 0.5 thus each S=O bond in the SO2 molecule is polar and has a specific dipole moment value.
The dipole moments of individual S=O bonds add up in the overall asymmetric shape of the SO2 molecule. The electron cloud stays non-uniformly distributed. So, the sulfur dioxide molecule is polar with net = 1.62 Debye.
Read in details
Also Check: How Does Australia’s Geography Affect Its Economy
Similarities Between Sulfur And Oxygen Atoms
So2 Molecular Geometry And Shape
To determine the molecular geometry of Sulfur Dioxide, we must observe its Lewis structure. There are two Oxygen atoms bonded to the central Sulfur atom. There is also a lone pair attached to the Sulfur atom. This indicated a bent molecular shape.
We can use the A-X-N concept and its table to verify and determine the molecular geometry of SO2.
A represents the central atom. The value of A here is 1.
X represents the number of atoms bonded to the central atom. In this case, there are two oxygen atoms bonded to the Sulfur atom.
Therefore, X =2.
N represents the number of lone pairs attached to the central atom. In this case, N = 1 as theres a lone pair of electrons attached to the Sulfur atom.
Therefore, that would give us AX2N for the SO2 molecule. From the A-X-N table below, we can determine the molecular geometry.
| Formula |
The AX2N formula corresponds to a bent molecular geometry.
Therefore, Sulfur Dioxide has a bent molecular geometry.
CONCLUDING REMARKS
Lets quickly summarize the salient features of the Sulfur Dioxide compound:
- SO2 comprises a Sulfur atom surrounded by two oxygen atoms.
- In its most stable state, the Sulfur atom forms double bonds with the adjacent oxygen atoms. There is also a lone pair above the Sulfur atom.
- The hybridization of the SO2 is given by sp2.
- SO2 has a Bent molecular structure and shape with bond angles of 120°.
About Priyanka
Also Check: College Algebra And Trigonometry Levitan
So2 Lewis Structure Hybridization Molecular Geometry And Mo Diagram
Ever wondered what causes the smell when matchsticks are burnt? Well, the answer is SO2!
SO2 is a very beneficial gas. Along with its main use, i.e sulfuric acid formation, SO2 has multiple functions in this chemical industry.
But before going through them, pause a little and give a read to this article. Because in the end, you will have deep knowledge about all the basics of SO2 you need to know, before moving on with the reactions.
So lets begin!!
Sulfur dioxide is spelled as Sulphur dioxide in Commonwealth English. This is a pungent-smelling, colorless gas.
Talking about its properties, SO2 has a molar mass of 64.066 g/mol. The melting point and boiling points are -72, and -10 respectively.
Now lets move on to the fundamental concepts like lewis structure, molecular geometry, MO Diagram, and hybridization of SO2.
Molecules With No Single Central Atom
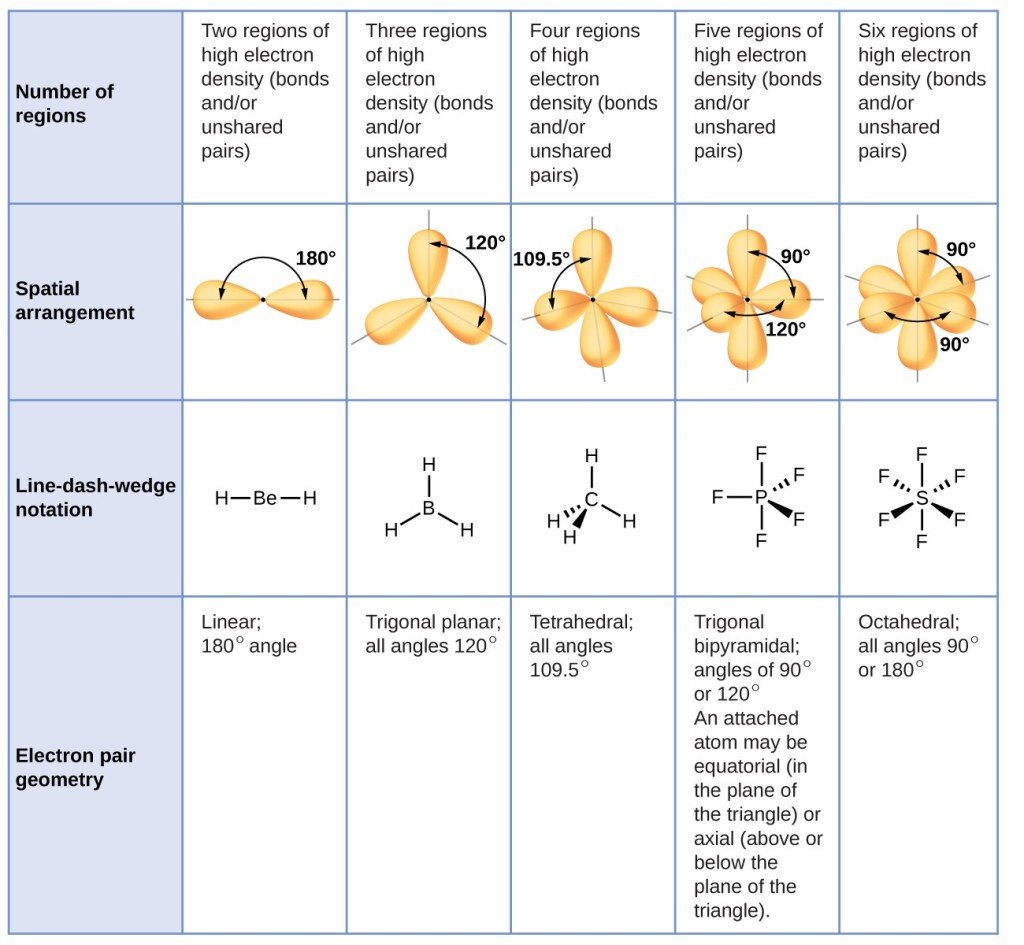
The VSEPR model can be used to predict the structure of somewhat more complex molecules with no single central atom by treating them as linked AXmEn fragments. We will demonstrate with methyl isocyanate , a volatile and highly toxic molecule that is used to produce the pesticide Sevin. In 1984, large quantities of Sevin were accidentally released in Bhopal, India, when water leaked into storage tanks. The resulting highly exothermic reaction caused a rapid increase in pressure that ruptured the tanks, releasing large amounts of methyl isocyanate that killed approximately 3800 people and wholly or partially disabled about 50,000 others. In addition, there was significant damage to livestock and crops.
We can treat methyl isocyanate as linked AXmEn fragments beginning with the carbon atom at the left, which is connected to three H atoms and one N atom by single bonds. The four bonds around carbon mean that it must be surrounded by four bonding electron pairs in a configuration similar to AX4. We can therefore predict the CH3N portion of the molecule to be roughly tetrahedral, similar to methane:
The nitrogen atom is connected to one carbon by a single bond and to the other carbon by a double bond, producing a total of three bonds, CN=C. For nitrogen to have an octet of electrons, it must also have a lone pair:
Figure 5.1.7 The Experimentally Determined Structure of Methyl Isocyanate
You May Like: Bernard Kolman Elementary Linear Algebra With Applications
So So2 Polar Or Nonpolar
Having calculated the difference in electronic potential, we know that the Oxygen-Sulfur bond is polar. Furthermore, the presence of a lone pair on the central sulfur atom and bent shape means that there is a lack of symmetry.
This then creates relatively more electronegative regions near the oxygen atoms. There is a net dipole moment, and thus the molecule is a polar one.
Therefore, SO2 is a polar molecule.
About Priyanka
To read, write and know something new every day is the only way I see my day! Well, that rhymed. Hey folks, this is me, Priyanka, writer at Geometry of Molecules where I want to make Chemistry easy to learn and quick to understand. Having an MSc degree helps me explain these concepts better. I write all the blogs after thorough research, analysis and review of the topics. And if not writing you will find me reading a book in some cosy cafe! View all posts by Priyanka
So2 Molecular Orbital Diagram
The molecular orbital diagram of SO2 is attached below:
A molecular orbital diagram gives us an idea about how the atomic orbitals of two different atoms can fuse and give rise to a new orbital.
This further helps us to find out the bond order, bond length, and bond strength of any compound.
In this MO we can see that the AO of sulfur, which is on the left-hand side associates with the AO of oxygen on the right-hand side.
We can see 18 electrons are filled in the orbitals with the proper rule.
There are certain non-bonding orbitals present there as well. Also, the antibonding orbitals are vacant in the situation of SO2.
This adds up to the explanation of the molecular orbital diagram of SO2.
You May Like: What Is Polymorphism In Biology
Dissimilarities Between Oxygen And Sulfur
Predict The Molecular Geometry And Polarity Of The So2 Molecule
Predict the molecular geometry and polarity of the SO2 molecule.
linear, polar
-
bent, polar bond angle is 119 degrees
Read Also: What Is Atomic Radius In Chemistry
Molecular Shape And Symmetry
Observing the Lewis structure and molecular shape will further help us determine whether SO2 is polar or non-polar. From the Lewis structure, it is evident that the molecule is vertically symmetrical. However, the presence of two lone pairs creates an imbalance in electronic distribution. This lone pair of electrons distorts the molecular geometry giving SO2 its Bent Molecular shape.
The presence of a lone pair indicates that the SO2 molecule is indeed polar.
What Is The Hybridization Of Sulphur Dioxide
In sulphur dioxide, the hybridization that takes place is sp2 type. To determine this, we will first look at the sulphur atom which will be the central atom. During the formation of SO2, this central atom is bonded with two oxygen atoms and their structure can be represented as O=S=O. As for the bonding, there is one sigma and one pi bond formed between sulphur and the two oxygen atoms. The atom will also accommodate one lone pair.
Let us break it down further. Looking at the ground state of sulphur consists of six electrons in the outermost shell and the first two shells are also completely filled. There are 4 electrons in the 3p portal and two paired electrons in the 3s orbital. Now, it has to form four bonds and thus needs 4 unpaired electrons. This leads to the development of the excited state in sulphur where one 3px electron jumps to the 3d orbital. When this happens there will be one unpaired electron in one 3d orbital and three in 3p orbitals. However, the electrons that form the sigma bonds and the lone pair are at different energy levels. The stable state is obtained when hybridization takes place.
Interestingly, the hybridization of the oxygen atom in this compound is also sp2.
Read Also: What Is The Definition Of Human Geography
Predicting Electron Pair Geometry And Molecular Structure
The following procedure uses VSEPR theory to determine the electron pair geometries and the molecular structures:
The following examples illustrate the use of VSEPR theory to predict the molecular structure of molecules or ions that have no lone pairs of electrons. In this case, the molecular structure is identical to the electron pair geometry.
Electronegativity And Bond Nature
A difference in electronegativity creates dipoles whereby electronegative forces dictate heavily. This makes the nature of the bond polar.
We look to the periodic table to determine the difference in charges between the Sulfur and Oxygen atoms. In this case the difference is 3.44 – 2.58 = 0.86.
This tells us that the Oxygen atoms are slightly more electronegative than the Sulfur atom, which is indicative of polar nature.
Now, we look to the Pauling scale to help verify. According to the Pauling scale, if the difference electronegativity lies between 0.5 and 2.0, the bonds are polar in nature.
Since that is the case above , we can safely say that the S-O bonds are polar.
Also Check: What Is Evaporative Cooling In Biology
So2 Lewis Structure Hybridization Molecular Geometry And Bond Angles
The chemical formula SO2 represents the chemical compound Sulfur Dioxide. The substance is a colorless gas with a recognizable pungent odor similar to the smell of a burnt matchstick.
A large quantity of SO2 is released during volcanic eruptions. It is also found in some hot water springs. Sulfur Dioxide contributes to global warming as a proponent of the greenhouse effect. This is evidenced by the sulfur cycle observed on Venus, where it forms clouds of Sulfuric acid. Trace amounts of the compound have also been observed on other bodies in the solar system.
Sulfur Dioxide is manufactured on an industrial scale by burning or roasting Sulfur and its components in the presence of Oxygen. The gas released is captured and primarily used in the production of Sulfuric Acid through the contact process. Here, SO2 is converted to Sulfur Trioxide, which combines with sulfuric acid to give Oleum . A combination of Oleum with water gives Sulfuric Acid. Billions of kilograms of SO2 are produced annually to meet global requirements.
In addition to its wide industrial use, SO2 is also used as a preservative in dried fruits, a reagent in the laboratory, and various biomedical applications. It is also being looked at as a potential refrigerant and as a tool for climate engineering.
As an irritant, it must be handled with care. Prolonged exposure can lead to respiratory illnesses.
SO2 has the following properties: