What Is A Portable Evaporative Cooler
A portable evaporative cooler essentially works off this principle by acting as a cooling fan that uses water, a pump, and a wetted surface to create the naturally occurring process of evaporation to cool warm air and drop temperatures. By pulling air across the water, the temperature of the air will be lowered.
One unique aspect of the evaporative cooler is that it actually works best with a supply of outside ambient air or even in an outside setting to deliver a temperature reduction and cooling effects. While evaporative coolers achieve significant temperature drops in more arid climates and drier spaces, they provide relief from the heat in any environment, even humid climates. For example, in regions where relative humidity reaches 70% at midday with temperatures higher than 90 degrees Fahrenheit, evaporative coolers have been shown to offer noteworthy relief. Given that relative humidity is lowest in the afternoon when the temperature is at its highest, an effective evaporative cooling scenario is achievable. In addition, evaporative cooling is extremely energy efficient, often using as little as $1 a day.
What Are The Steps Of Evaporation
In the water cycle, there are four key phases. Evaporation, condensation, precipitation and collection are as they are. Lets look at the steps of both of these. Evaporation: This is where the suns heating induces water to rise into the air from seas, lakes, streams, glaciers and soils, and transform into water vapour .
Iicurrent Understanding Of The Stbl Regime
Stratocumulus often occur over the subtropical oceans off the west coasts of the major continents throughout most of the northern summertime. Their favorite large-scale condition is strong subsiding motion over a cold ocean surface. This large-scale condition creates a very moist, shallow PBL. The lifting condensation level is below the top of the mixed layer so clouds that form in the upper part of the PBL are likely to spread out uniformly in the horizontal direction due to turbulent mixing. This cloud regime typically covers tens of thousands of square kilometers and, thus, can easily fill a few GCM grid meshes horizontally. Vertically, this cloud layer is often 100500 m thick and thus can hardly be well resolved by a GCM vertical grid.
APhysical Processes
Figure 1. A sketch showing the various physical processes involved in the stratocumulus-topped PBL.
Entrainment brings in not only warm air but also dry air. Entrained warm air that mixes in with the radiatively cooled near-cloud-top air can make downdrafts warmer and hence decrease the cloud-top buoyancy. On the other hand, entrained dry air that mixes with cloudy air results in evaporation, which cools unsaturated downdrafts near the cloud top more rapidly and hence enhances the cloud-top buoyancy forcing.
BTypical Profiles of the Thermodynamical Fields
Figure 2. Typical profiles of the mean total mixing ratio, the mean liquid water potential temperature, and the mean potential temperature within a well-mixed STBL.
Don’t Miss: What Are 4 Goals Of Psychology
Maximum Yield Explains Evaporative Cooling
There are two ways to achieve evaporative cooling in a greenhouse or growroom. One way uses a cabinet cooler, which is often referred to as a swamp cooler, and the other way uses a fan-and-pad system.
A cabinet cooler relies on a thermostat to monitor the temperature. When the temperature reaches the pre-set high, the thermostat triggers the cabinet cooler to turn on and immediately start the evaporative cooling process in the greenhouse. Once the temperature dips to the desired level, the thermostat tells the cabinet cooler to turn off. A cabinet cooler can also be controlled manually by the grower and turned on or off as desired.
The fan-and-pad system, which has been the standard method for decades, also commonly relies on a thermostat to start the evaporative cooling process. If a high-tech thermostat is not available, then the grower can control the system by turning it on and off manually when needed. With this system, aspen or cellulose pads are mounted on a sidewall of the greenhouse. The pads are fed water from attached pipes so they are constantly kept wet. Air is then drawn through the wet pads by fans on the opposite wall of the greenhouse, and the process creates a cooling vapor.
Related Question
Thermal Properties Of Water

Water is the universal solvent and indispensable for life. From the elements in the climate-space equation, it is obvious that the water balance, through evaporative cooling, can affect the organism’s temperature. Therefore, it is not surprising that the temperature relations of many organisms with their environment are closely connected to their water balance. The thermal properties of water are important in determining the thermal relations of organisms. The heat conductivity of water is 0.0014 cal cm1 sec1 C1, which is low compared to that of other materials such as metals, but is higher than most other common solvents, e.g., olive oil, 0.000393, or ethyl alcohol, 0.00042 cal cm1 sec1 C1. Consequently, water as a solvent allows the organism to remain partially coupled to its thermal environment in such a way as to permit its metabolic processes to reflect thermal regimes of the environment, but sufficiently meliorated so as to permit temporary exploitation of marginal habitats.
Recommended Reading: Kuta Infinite Geometry
How Does Evaporative Cooling Work
Evaporative cooling, otherwise known as adiabatic cooling, works on the principle of water evaporation through which the air is cooled down to a comfortable temperature.
It is a cooling and ventilation technique that uses water as its refrigerant.
During the evaporative cooling process, water is evaporated in a stream of air and passes from a liquid to a gas. This transition requires energy, which is extracted from the air in the form of heat. As a result of this process, the air is cooled down.
This evaporative cooling process can be applied in several ways:
- Indirect/direct adiabatic cooling
- Indirect adiabatic cooling
For a clear overview of what these different methods entail, check out our page about two stage evaporative cooling.
Find The Best Examples Of Evaporation Described By The Experts
In the previous classes, you have studied about three natural phenomena, evaporation, condensation, and vaporization. In this concept page, we will dig a little deeper to find out the underlying physical reasons behind these phenomena. This part of the chapter will concentrate on the evaporation and condensation part. The concept page has been developed by the top experts of Vedantu so that every student can easily grab hold of the basic Physics behind these phenomena and answer the questions perfectly.
This is an important part of chapter matter. These concepts will become a mandatory part of the physical properties of different elements and compounds in the advanced chapters. Here, evaporation and condensation will be properly discussed so that the students can understand their meaning and find the basic differences between them. Follow this concept page as a reference and study the chapter properly to overcome your doubts and answer the questions easily.
Don’t Miss: Algebra 1 Eoc Answers 2015
Evaporative Cooling System: How Does It Work
In an evaporative cooling system, hot outside air is forced through wet cooling pads by means of a motor-driven fan. The cooling pads are moistened continuously by a water pump that delivers water to the cooling pads. The cooled down air is then blown into the building. The outcoming air can then be cooled down between 60 and 90 % of the wet-bulb depending on the effectiveness of the evaporative media. The outcoming air is cooled down 10 to 15 °C but contains a high amount of humidity. Therefore direct evaporative cooling is not recommended for cooling in work and living environments.
Two-stage evaporative cooling, on the other hand, produces efficiencies up to 114% of the wet bulb, resulting in temperatures up to 7 °C lower, and due to the lower temperature, it contains 60% less humidity than direct evaporative cooling processes.
Check out our two-stage adiabatic cooling system on the IntrCooll page.
Latent And Sensible Heat
The fundamental governing process of evaporative cooling is heat and mass transfer due to the evaporation of water. This process is based on the conversion of sensible heat into latent heat. Sensible heat is heat associated with a change in temperature. While changes in sensible heat affect temperature, it does not change the physical state of water. Conversely, latent heat transfer only changes the physical state of a substance by evaporation or condensation .
As water evaporates, it changes from liquid to vapor. This change of phase requires latent heat to be absorbed from the surrounding air and the remaining liquid water . As a result, the air temperature decreases and the relative humidity of the air increases. The maximum cooling that can be achieved is a reduction in air temperature to the wet-bulb temperature at which point the air would be completely saturated .
Also Check: Who Are Paris Jackson’s Biological Parents
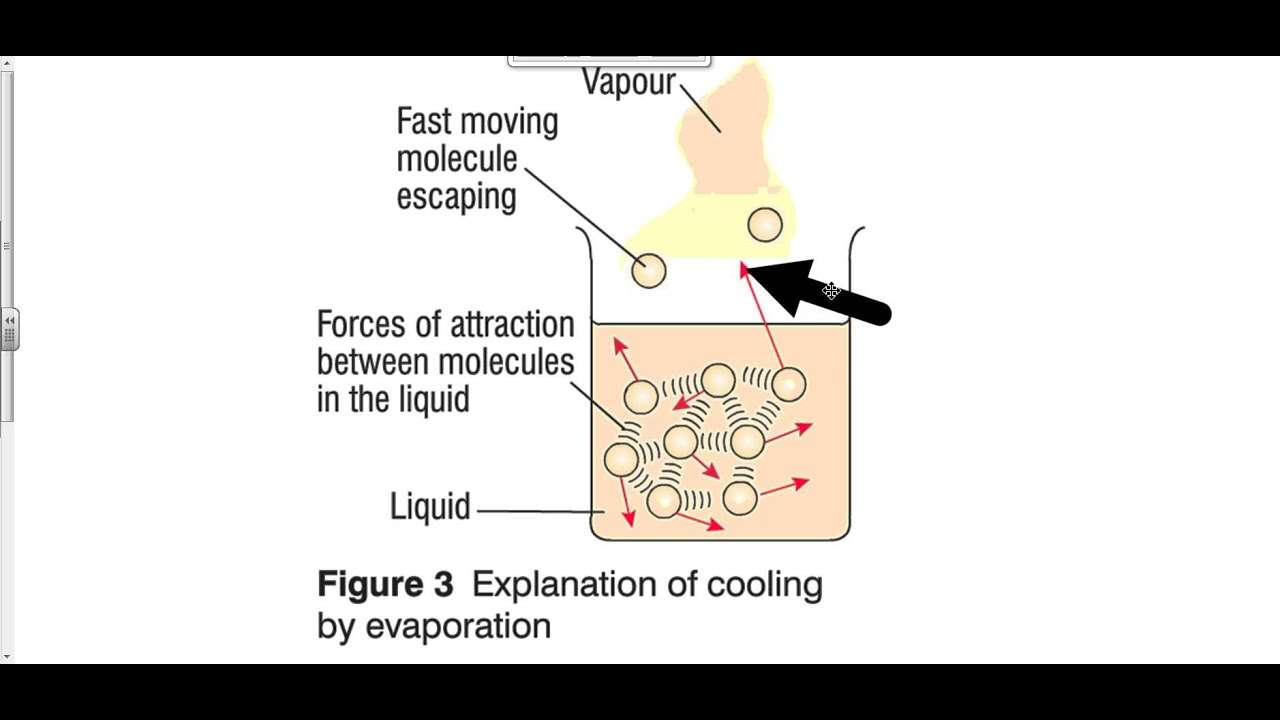
Meaning And Definition Of Evaporative Cooling :
The property of a liquid whereby the surface becomes cooler during evaporation, owing to a loss of highly kinetic molecules to the gaseous state.
For the term evaporative cooling may also exist other definitions and meanings, the meaning and definition indicated above are indicative not be used for medical and legal or special purposes.
Source : SFU Text file : http://school.gogpg.com/Portals/1/Assess%20Well/Example%20Sampling%20Domains-Curriculum%20Specific.xls
If you are the author of the text above and you not agree to share your knowledge for teaching, research, scholarship please send us an e-mail and we will remove your text quickly.
Fair use is a limitation and exception to the exclusive right granted by copyright law to the author of a creative work. In United States copyright law, fair use is a doctrine that permits limited use of copyrighted material without acquiring permission from the rights holders. Examples of fair use include commentary, search engines, criticism, news reporting, research, teaching, library archiving and scholarship. It provides for the legal, unlicensed citation or incorporation of copyrighted material in another author’s work under a four-factor balancing test.
Google key word : evaporative cooling
Types Of Evaporative Coolers
Evaporative cooler designs vary based on absorbent medium, storage chamber construction, method of evaporation. Many different evaporative cooler designs exist in both unpowered and externally powered forms. Evaporative coolers that need no external power are designed to allow for natural airflow to provide the convection needed for adequate cooling. Various designs within this group allow for heat transfer in slightly different ways. While all coolers rely on converting sensible heat to latent heat, the mechanism for this conversion can be different.
Some evaporative cooler designs are created using porous materials to allow water to seep from an inside container to an outer surface where evaporation occurs. For these designs, evaporation is aided by drawing heat from the inner cooling chamber. As warmer water molecules evaporate and leave the outer surface, the surface cools and a heat gradient causes heat to diffuse from the inner chamber out to the surface. As a result, the temperature within the inner chamber decreases causing a cooling effect on whatever is stored in the cooler.
Unpowered
Externally Powered
\displaystyle = \frac\ }
Other Types
Other alternative evaporative cooling methods include fog or misting systems, which spray small diameter water droplets into the air for evaporation, and roof top evaporation systems which involve a thin water layer on the top of a building resulting in evaporation and cooling
Recommended Reading: Who Is Paris Jackson’s Real Father
Engine And Test System
It is well known that Gasoline Direct Injection engines can operate with a higher compression ratio than Port Fuel Injection engines because the evaporative cooling from the fuel can be used to cool the incoming air. Since the temperature is lower at the start of compression, then all subsequent processes will be at a lower temperature, so for a given octane rating fuel, then a higher compression ratio can be used for a given charge temperature at the end of compression. The lower charge temperature also increases the volumetric efficiency leading to an increase in the specific output and a consequential additional reduction in fuel consumption. The increased enthalpy of vaporisation with ethanol blends could lead to additional gains, but mixtures might also be less homogeneous.
Table 2. Single-cylinder GDI optical engine specification
| Combustion Injector | |
| Manifold Absolute Pressure | 0.5 bar |
A series of injection and combustion images was captured by a Photron PCI-1024 high speed video camera at up to 9,000 fps. Illumination of the spray was with a synchronized LED system, whilst the combustion process was visualized with the visible light flame chemiluminescence. Image processing was used to separate the spray from the background and the pixel count within the spray was integrated to give a semi-quantitative value for the Mie scattering.
Juan M. Salazar, Urmila M. Diwekar, in, 2012
Tio2 Photocatalyst Evaporative Shell
Evaporative cooling may also be exploited by spraying a continuous water layer on building surfaces to cool them by subtracting heat via its evaporation. A recent innovative method takes advantage of a TiO2 photocatalyst, which when irradiated by the sun causes the surface to become highly hydrophilic, minimizing the amount of water consumption to form the water film. This way it is possible to cover the whole building with little water supply, as the water layer is just 0.1 mm thick. Testing showed a temperature drop of 15°C on window glass and 4050°C on black roof-tile surfaces on a clear day in the middle of summer, a promising result that could significantly reduce electricity consumed for air-conditioning or avoid its need altogether.21
X. Zhao, in, 2010
Read Also: Ccl4 Lone Pairs
How Does Evaporation Cause Cooling
- Evaporation causes cooling naturally. The underlying principle behind this is, in order to change its state, the matter must either gain or lose energy. In the case of change of phase from liquid to gas, molecules of matter require energy to overcome their potential energy by their kinetic energy. So, the liquid takes this energy from its surroundings.
- Generally, when energy transfer occurs, it results in an increase or decrease in temperature of the substance, depending on whether the energy is being transferred from the substance to the surroundings or vice versa. However, there are exceptions to this rule.
- Although there is an increase in temperature of the substance until the boiling point is attained during evaporation, phase change results in no observable heat transfer.
- The molecules of the substance absorb heat energy continuously from the surroundings and thus cool the surroundings until they reach the boiling point, after which they start to break free from the liquid and turn into vapour. Since there is no change in temperature till the evaporation process is complete i.e. the entire liquid gets converted into vapour, the amount of energy required for this phase change is called the latent heat of vaporization, where the word latent means hidden, meaning this heat will not change the temperature reading on a thermometer.
The Reason For Everything
Read Also: What Happened To Jonathan Thomas Child Of Rage
Evaporative Cooling Systems: How And Why They Work
Many poultry houses today are equipped with cooling systems that consist of an arrangement with cool cell pads at one end of the house and large tunnel exhaust fans at the other end. To master operation of their evaporative cooling systems, poultry growers must have a working understanding of the relationship between temperature and humidity and the effect it has on chickens. The relationship is both simple and complicated.
The simple part is that as temperature goes up, humidity goes down and vice versa. That relationship is quite linear, and it works well. Consider the weather in Phoenix, Arizona, on a sunny summer afternoon. It may be 110 °F, but, as they say, its a dry heat. There is almost no moisture in the air when the temperature is that hotoften its less than 10 percent humidity. As a result, evaporative cooling works great in the desert.
Now consider the weather on your farm on a summer morning at sunrise. Its around 70 °F and the humidity is at or near 100 percent. Dew formed overnight, and water is dripping off the chicken house roof. Why? The air temperature dropped overnight. Because cold air holds less moisture, the air became saturated and condensation formed on surfaces.
What Is Evaporative Cooling
Evaporative cooling is a cooling technique which uses the evaporation of water to lower the temperature. Humans actually use evaporative cooling naturally when they sweat as they heat up, and the sweat evaporates, lowering body temperature. The same principle can be used to bring the air temperature down in warm climates. This type of cooling is generally only suitable for hot, dry climates. In a humid climate, evaporative cooling cannot work as well, and it can cause the humidity to reach an uncomfortable level.
The basic design of an evaporative cooler consists of a fan which pulls hot air through a series of water soaked pads. As the air moves through the pads, it cools down, and the cool air can be vented directly into a room or into a duct system. The humidity will also increase slightly, thanks to the water in the air, which can actually be beneficial in very dry climates where the dry air leads to issues like cracked lips and dry skin.
Ever since she began contributing to the site several years ago, Mary has embraced theexciting challenge of being a HomeQuestionsAnswered researcher and writer. Mary has a liberal arts degree from Goddard College andspends her free time reading, cooking, and exploring the great outdoors.
Recommended Reading: Jonathan Thomas Beth Thomas