Physical Chemical Characteristics Of Fluorine
Table 1. Physicalchemical data of fluorine
| Melting point |
| O2+ | N2+ |
On March 23, 1962, when platinum hexafluoride, a red gas, was allowed to mix with a large molar excess of xenon , the immediately formed product was a yellow solid with the composition Xe+ , the first synthesized compound of a noble gas.99
Fig. 27. Synthesis of the first compound of noble gases by N. Bartlett: Xe and PtF6 gases prior to reaction resulting XePtF6.
This discovery was acclaimed101 as one of the ten most beautiful experiments in chemistry, because102 it opened the way for the noble gas chemistry and high oxidation species chemistry, as illustrated by the various species isolated in Neil’s Berkeley Group: first example of Os + VII: OsOF5 NOWF7, 2WF8, NOReF7 Isolation of ONF3: 2NiF6 ONF + ONF3 + NiF2 synthesis and oxidizing properties of ReOF5 and OsOF5 salts of quinquevalent gold: M+ AuF6, NO+ AuF6, Xe2F11+ AuF6, XeF5+ AuF6, etc. Quoting P. Ball, we can say that Neil Bartlett’s experiment tells us that in chemistry, the wonders never cease.103
Now, fluorine gas is produced following the electrochemical process, as shown, for instance at Fig. 28 for the F2 synthesis facilities of Advance Research Chemicals, Inc. . An important technical point is that hooded areas should be specially equipped for fluoride manufacturing. All work should be done in hoods, 40,000 cfm fresh air being pulled in to avoid depression in the building.
Fig. 28. Fluorination facilities at ARC, Inc.
Pressure Units And Equivalencies
- 2116.22pounds-force per square foot
- = 1 ata .
The ata unit is used in place of atm to indicate the total pressure of the system, compared to a vacuum. For example, underwater pressure of 3 ata would mean that this pressure includes 1 atm of air pressure and thus 2 atm due to the water.
What Does Atm Mean On A Watch
ATM table above shows the required ATM by activity. ATM is simply an abbreviation of the word ATMOSPHERE and this relates the the amount of pressure a watch is designed to endure, pressure increases the deeper you go underwater.
On our website, we group watches into 3 categories of water resistance these are splash-proof, waterproof and dive.
Also Check: What Are Physical Features In Geography
The Ideal Gas Equation
Before we look at the Ideal Gas Equation, let us state the four gas variables and one constant for a better understanding. The four gas variables are: pressure , volume , number of mole of gas , and temperature . Lastly, the constant in the equation shown below is R, known as the the gas constant, which will be discussed in depth further later:
Another way to describe an ideal gas is to describe it in mathematically. Consider the following equation:
The term \ is also called the compression factor and is a measure of the ideality of the gas. An ideal gas will always equal 1 when plugged into this equation. The greater it deviates from the number 1, the more it will behave like a real gas rather than an ideal. A few things should always be kept in mind when working with this equation, as you may find it extremely helpful when checking your answer after working out a gas problem.
- Pressure is directly proportional to number of molecule and temperature.
- Pressure, however, is indirectly proportional to volume.
Key Takeaways: Stp Or Standard Temperature And Pressure
- STP is the abbreviation for Standard Temperature and Pressure. However, the “standard” is defined differently by various groups.
- STP values are most often cited for gases because their characteristics change dramatically with temperature and pressure.
- One common definition of STP is a temperature of 273 K and the standard pressure of 1 atm. Under these conditions, one mole of a gas occupies 22.4 L.
- Because the standard varies by industry, it’s good practice to state temperature and pressure conditions for measurements and not just say “STP.”
You May Like: What Does G Mean In Physics
Reaction Order And Rate Constant Units
In some of our examples, the reaction orders in the rate law happen to be the same as the coefficients in the chemical equation for the reaction. This is merely a coincidence and very often not the case.
Rate laws may exhibit fractional orders for some reactants, and negative reaction orders are sometimes observed when an increase in the concentration of one reactant causes a decrease in reaction rate. A few examples illustrating these points are provided:
It is important to note that rate laws are determined by experiment only and are not reliably predicted by reaction stoichiometry.
Reaction orders also play a role in determining the units for the rate constant k. In Example 2, a second-order reaction, we found the units for k to be \text^\text^, whereas in Example 3, a third order reaction, we found the units for k to be mol2 L2/s. More generally speaking, the units for the rate constant for a reaction of order are \text^\text^\text^.Table 7 summarizes the rate constant units for common reaction orders.
| Reaction Order |
|---|
| mol2 L2 s1 |
| Table 7. Rate Constants for Common Reaction Orders |
Note that the units in the table can also be expressed in terms of molarity instead of mol/L. Also, units of time other than the second may be used, depending on the situation.
What Is Waterproofness Exactly How Is It Determined
The watches are made with mechanical and electronic parts that can be damaged by water and humidity In this case, the waterproofness of the watch can be defined as its resistance to water pressure. When you buy a watch, request information on its waterproofness to avoid future disappointment. Waterproofness is determined in a laboratory under perfect conditions, that is, at constant temperature with the watch immobile.
Read Also: What Is Imprinting In Biology
Whats The Difference Between An Agm And A Regular Car Battery
AGM car batteries have unbeatable advantages over standard, flooded batteries:
- More starts per battery
- Safer to handle
- Special valves protecting the battery’s lifespan
Over the course of their lifespan, AGM batteries can start an engine more than 60,000 times. Thats more than three times the starts youll get out of a conventional battery.
And AGMs recharge faster than typical batteries. Starting your engine depletes your battery only a small amount before the alternator takes over. When it does, the alternator recharges the battery and keeps all the electrical components running in the car.
Because of their absorbed mats, AGMs withstand shaking and vibration better than typical batteries. Theyre also listed as spill-proof, meaning the regulations are more relaxed about transporting them by air or by road.
This might sound like marketing hype. Instead, its just science.
Heres how AGMs work.
Gas Constant In Chemistry
- In chemistry, the gas constant goes by many names, including the ideal gas constant and universal gas constant.
- It is the molar equivalent to the Boltzmann constant.
- The SI value of the gas constant is exactly 8.31446261815324 JK1mol1. Usually, the decimal is rounded to 8.314.
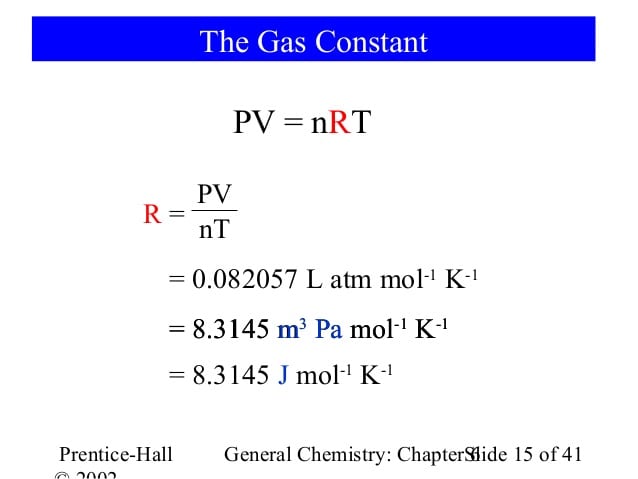
The Gas Constant is the physical constant in the equation for the Ideal Gas Law:
- PV = nRT
P is pressure, V is volume, n is the number of moles, and T is temperature. Rearranging the equation, you can solve for R:
R = PV/nT
The gas constant is also found in the Nernst equation relating the reduction potential of a half-cell to the standard electrode potential:
- E = E0 – lnQ
E is the cell potential, E0 is the standard cell potential, R is the gas constant, T is the temperature, n is the number of mole of electrons exchanged, F is Faraday’s constant, and Q is the reaction quotient.
The gas constant is equivalent to the Boltzmann constant, just expressed in units of energy per temperature per mole, while the Boltzmann constant is given in terms of energy per temperature per particle. From a physical standpoint, the gas constant is a proportionality constant that related the energy scale to the temperature scale for a mole of particles at a given temperature.
Units for the gas constant vary, depending on other units used in the equation.
Recommended Reading: Which Csu Has The Best Biology Program
Key Concepts And Summary
Rate laws provide a mathematical description of how changes in the amount of a substance affect the rate of a chemical reaction. Rate laws are determined experimentally and cannot be predicted by reaction stoichiometry. The order of reaction describes how much a change in the amount of each substance affects the overall rate, and the overall order of a reaction is the sum of the orders for each substance present in the reaction. Reaction orders are typically first order, second order, or zero order, but fractional and even negative orders are possible.
Understanding Standard Temperature And Pressure
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
STP in chemistry is the abbreviation for Standard Temperature and Pressure. STP most commonly is used when performing calculations on gases, such as gas density. The standard temperature is 273 K and the standard pressure is 1 atm pressure. This is the freezing point of pure water at sea level atmospheric pressure. At STP, one mole of gas occupies 22.4 L of volume .
Don’t Miss: What Is Atp Stand For In Biology
How Often Should I Have My Watch Checked For Waterproofness
When the watch has been opened for servicing, make sure that all waterproofness checks have been done, especially if you use the watch in water or in a damp environment on a regular basis. We recommend that you have the waterproofness of your watch checked once a year and every time the watch is opened.
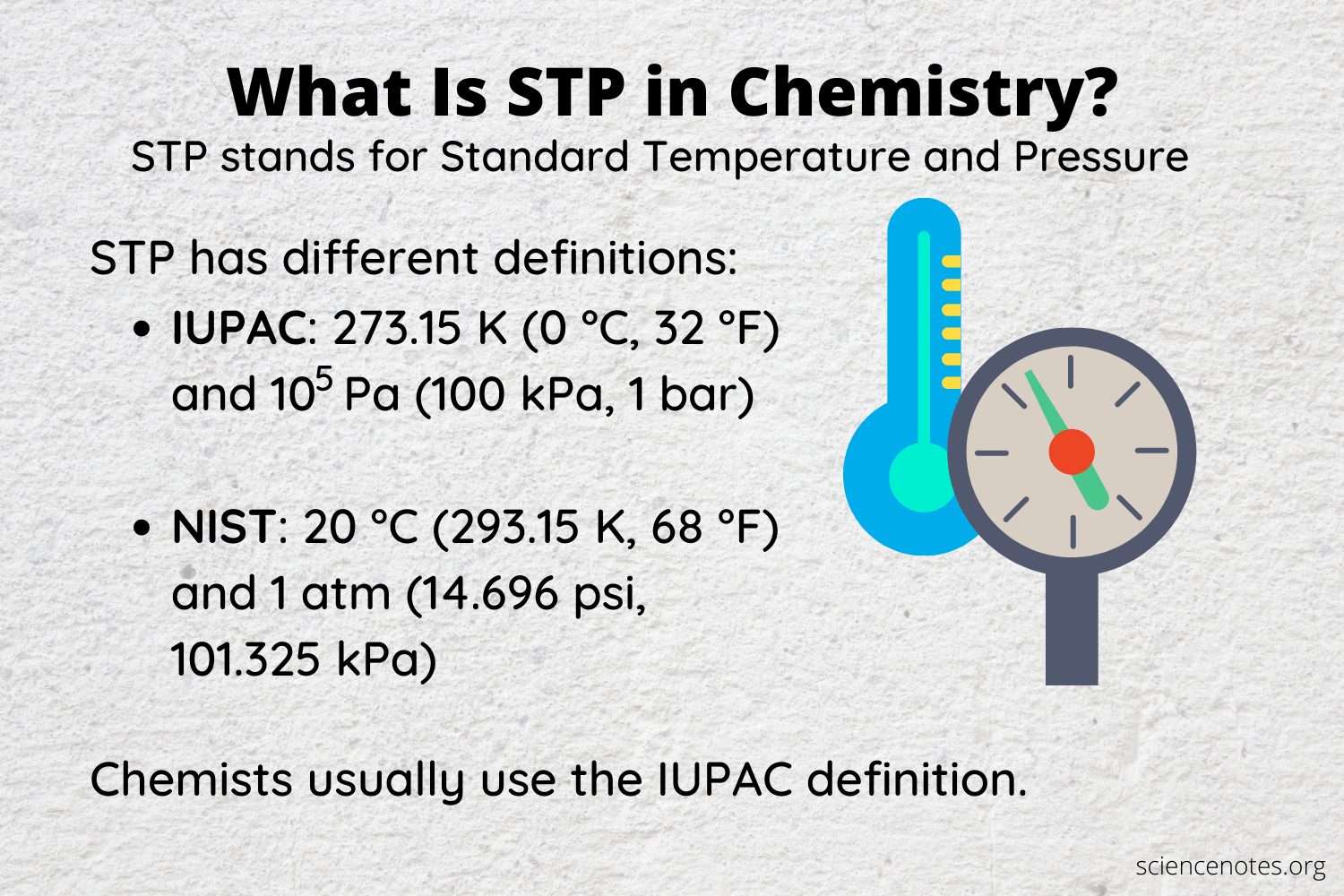
Definitions Of Standard Temperature And Pressure
The term STP means different things to different people and can cause problems in the presentation of adsorption data, because the most common units for the ordinates of such plots are standard volumes per unit mass of adsorbent.
The International Union of Pure and Applied Chemistry used to define STP as 0 C and 1 atm . This definition is now obsolete. The preferred definition, since 1982, is 273.15 K and 1 bar . The National Institute of Standards and Technology, on the other hand, defines STP as 1 atm and 20 C . In practical applications of adsorption equipment, STP often refers to the pressure of the room and the temperature of the adsorption manifold, which is often kept above room temperature to prevent changes in room temperature from changing the apparent manifold or dead volumes . Room temperature, at least in the summer months in many places, is usually close to 25 C, which is a de facto standard in gas flow controllers.
For the sake of comparison, we recommend specifying the definitions of STP being used if there is any concern that they will affect the results. The difference between using a standard temperature of 0 C and 25 C will, in the authors’ experience, produce errors in the volume adsorbed that are less than the error in the measurements themselves. In commercial equipment, the volume adsorbed is usually reported by defining STP as 0 C and 760 Torr .
Alain Tressaud, in, 2019
Also Check: What Is Demagnetisation In Physics
What Is Atm Implied Volatility
A tool that measures the calculated or implied mid-rate volatility for an ATM option for a specific expiration date. Using the Black-Scholes model the ATM volatility can be defined as the volatility value that makes the implied price of an ATM vanilla option equal to the market price of that option.
Atm Meaning In Chemistry
- And finally again and again search .
Also Check: What Is Conceptual Understanding In Math
How Do You Find Atm In Chemistry
There is no one definitive answer to this question. Different chemists may use different methods to find atm in chemistry, but some general tips include using a gas chromatograph or a mass spectrometer to measure the concentration of molecules in a sample, and using a gas chromatograph to measure the presence of gas molecules.
Atm Full Form In Networking
ATM: Asynchronous Transfer Mode
Asynchronous Transfer Mode is a telecommunications standard used by telephony , data and video signals across a network without the use of separate overlay networks.
ATM Ka Full Form
What is ATM Means?
The full name of ATM is Automatic Teller Machine, which is used for money transactions.
What Does ATM Stand For?
ATM Full Form is Automated Teller Machine.
Social Media Links
Also Check: What Is The Basic Unit Of Chemistry
Chemistry End Of Chapter Exercises
What is the order of the reaction with respect to that reactant?
Tripling the concentration of a different reactant increases the rate of a reaction three times. What is the order of the reaction with respect to that reactant?
What is the order of the reaction with respect to that reactant?
Increasing the concentration of a reactant by a factor of four increases the rate of a reaction four times. What is the order of the reaction with respect to that reactant?
Increasing the concentration of CO from 0.01 M to 0.03 M.
Increasing the pressure of NO2 from 0.1 atm to 0.3 atm
Increasing the concentration of CO from 0.02 M to 0.06 M.
Determine the rate equation, the rate constant, and the overall order for this reaction.
Why Do We Need Standards
Chemists require STP definitions because the behavior of a substance varies greatly depending on the temperature and pressure. STP definitions give chemists a common reference point to describe how a gas behaves under normal conditions. Scientists use standards like STP definitions for two purposes, to define certain quantitative metrics and to allow for consistent and repeatable experiments.
Imagine someone tells you the molar volume of methane is 22.4 liters . The molar volume of a substance is just a measure of how much space one mole of that substance takes up. On its own, this value is not very informative. It is known that the volume of a gas varies greatly with respect to pressure and temperature, so gas could have multiple molar volumes, depending on the exact temperature and pressure. One needs to specify a temperature and pressure to make a molar volume measurement of 22.4 L a more meaningful quantity. Scientists agree upon a predefined temperature and pressure to report quantitative properties of gases. As it just so happens, one mole of any gas at STP has a volume of 22.4 L. Quantitative measurements of gas, like volume, volumetric flow, and compressibility, all must be defined with respect to some defined pressure and temperature.
The true method of knowledge is experiment. William Blake
Also Check: Delta Math Answers Algebra 2
What Is Stp In Chemistry
Standard temperature and pressure refers to the internationally agreed-upon standard of measurement for experiments in chemistry.
According to the International Union of Pure and Applied Chemistry , the currently accepted values for standard temperature and pressure are 273.15 K and exactly 100kPa . The purpose of STP is to provide chemists with a common experimental baseline from which to interpret and compare data.
All life is an experiment. The more experiments you make, the better. Ralph Waldo Emerson
The IUPAC definitions of STP are not universally accepted. Most chemistry textbooks, for example, still use a standard pressure value of 1 atm. Different industries use different standards, depending on what exactly their interests are. For instance, the National Institute of Standards and Technology defines STP to be 293.15 K and 1 atm of pressure , and the International Standard Metric Conditions defines STP to be 288.15 K and 101.325 kPa. Although the exact values of STP might change from context to context, the underlying idea is the same STP values provide a commonly agreed-upon set of experimental conditions in which to observe and describe the behavior of substances.
Calculations Relating To Volume And Calibration
At S.T.P. the volume of oxygen in 1.0 ml of atmospheric air is 210 l. Since one mole of gas occupies 22.414 1 , this volume of O2 contains 210/22.414 or 9.37 mol. At any other temperature , the amount of oxygen can be obtained by multiplying by 273/ thus at 20°C the corresponding value is 8.73 mol. Because the oxygen electrode measures concentration it is also necessary, for purposes such as this, to know the effective volume of the chamber itself. This, of course, is variable because the leaf disc and any other material enclosed within the fixed volume of the chamber will occupy a significant space. However, the increase in signal from the electrode circuit can be used to calculate both the effective volume of the chamber and the linearity of the electrode response. Suppose, for example, that the initial electrode output was 1.5 mV and that, after the plunger was fully depressed, this reading had increased to 1.8 mV . The effective volume of the chamber can be calculated from the relationship:
can also be used if sufficiently large excursions result from the introduction of volumes of air smaller than 1.0 ml.
where: VD = anatomical dead space and VT = respiratory tidal volume .
4.13. Rates of COHb formation at different VE levels for a constant 4000 ppm exposure concentration calculated using the CFK equation assuming a constant DLCO of 30 ml/min/mmHg.
Thomas M. York, Hai-Bin Tang, in, 2015
Don’t Miss: How Can I Apply Psychology To My Future Life