Confusion Regarding Work And The First Law Of Thermodynamics
I am a bit confused about an aspect the “work” part in the first law of thermodynamics, which says that the change in the internal energy of a system is the work done on the system + the heat transferred to the system. Here’s my question:
If I do work on, say, a stone, causing it to gain a large total kinetic energy, then according to the first law of thermodynamics , the internal energy has increased. But internal energy simply means the energy contained within the stone, not external energies, such as gravitational potential energy, or, more importantly in this case, the overall kinetic energy of the system. How is the first law of TD consistent with this definition of internal energy? The same problem arises, if, say, I also raise a stone by doing work on it, thus increasing the gravitational potential energy.
- 3$\begingroup$Together with ValterMoretti’s answer below, I recommend that you check some good textbook that explains the first law in the broader perspective of mechanics. Check for example the first chapter in Astarita’s Thermodynamics: he explains in detail the relationship between the action of forces, work, kinetic energy, internal energy, heat, with examples.$\endgroup$
Human Metabolism And The First Law Of Thermodynamics
Human metabolism is the conversion of food into heat transfer, work, and stored fat. Metabolism is an interesting example of the first law of thermodynamics in action. We now take another look at these topics via the first law of thermodynamics. Considering the body as the system of interest, we can use the first law to examine heat transfer, doing work, and internal energy in activities ranging from sleep to heavy exercise. What are some of the major characteristics of heat transfer, doing work, and energy in the body? For one, body temperature is normally kept constant by heat transfer to the surroundings. This means Q is negative. Another fact is that the body usually does work on the outside world. This means W is positive. In such situations, then, the body loses internal energy, since U = Q W is negative.
Now consider the effects of eating. Eating increases the internal energy of the body by adding chemical potential energy . The body metabolizes all the food we consume. Basically, metabolism is an oxidation process in which the chemical potential energy of food is released. This implies that food input is in the form of work. Food energy is reported in a special unit, known as the Calorie. This energy is measured by burning food in a calorimeter, which is how the units are determined.
Can You Break The First Two Laws Of Thermodynamics
To break the first law of thermodynamics, wed have to create a “perpetual motion” machine that worked continuously without the input of any kind of power. That doesnt exist yet. All the machines that we know receive energy from a source and transform it into another form of energy. For example, steam engines convert thermal energy into mechanical energy.
To break the first law of thermodynamics, life itself would have to be reimagined. Living things also exist in concordance with the law of conservation of energy. Plants use photosynthesis to make food and animals and humans eat to survive.
Eating is basically extracting energy from food and converting it into chemical energy which is what actually gives us energy. We turn that chemical energy into mechanical energy when we move, and into thermal energy when we regulate our bodys temperature, etc.
But things may be a bit different in the quantum world. In 2002, chemical physicists of the Australian National University in Canberra demonstrated that the second law of thermodynamics can be briefly violated at the atomic scale. The scientists put latex beads in water and trapped them with a precise laser beam. Regularly measuring the movement of the beads and the entropy of the system, they observed that the change in entropy was negative over time intervals of a few tenths of a second.
This is a real-life demonstration of Maxwell’s demon, a thought experiment to break the second law of thermodynamics.
You May Like: A Drastic Way To Diet Algebra With Pizzazz
First Law Of Thermodynamics Definition
| Working Of A Thermal Power Station Is Based On 1st Law Of ThermodynamicsCredit: Wikimedia Commons |
The First Law Of Thermodynamics is one of the Physical Laws Of Thermodynamics that states that heat is a form of energy and the total energy of a system and its surrounding remained conserved or constant. Or in more simple terms, for an isolated system energy can neither be created nor be destroyed. It can only be converted from one form of energy to another. Thats why the 1st law of thermodynamics is also known as the Law Of Conservation Of Energy. This law was discovered by German mathematician and physicist Rudolf Clausius.
see also, Gay-Lussacs Law Of Thermodynamics The Law Of Constant Volume
Perpetual Motion Machines Of First Kind
Second Law Of Thermodynamics
Irreversible or spontaneous processes can occur only in that direction for which the entropy of the universe or that of an isolated system, increases. These processes cannot occur in the direction of decreasing entropy.
For an isolated system,
Get the Magnetic Effect of Electric Current in detail.
Don’t Miss: What Does The Letter A Mean In Math
Explaining The First Law Of Thermodynamics
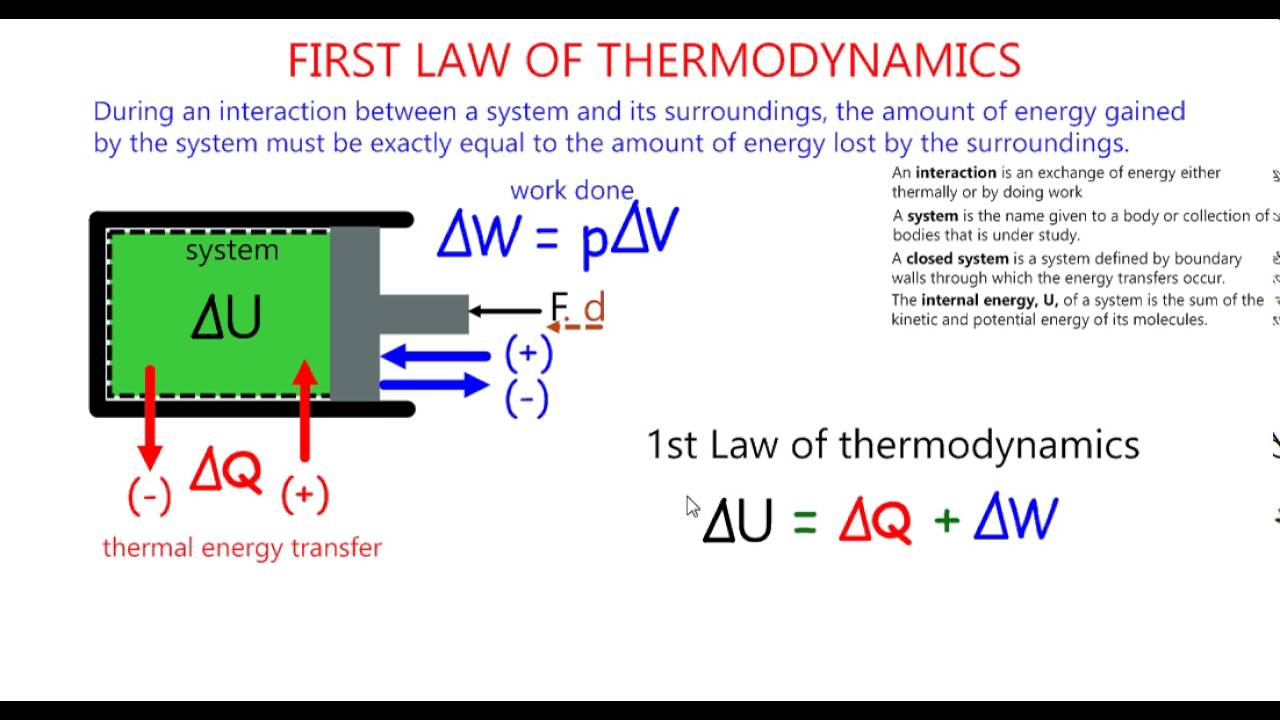
The first law of thermodynamics relates to heat, internal energy, and work.
The first law of thermodynamics, also known as the law of conservation of energy states that energy can neither be created nor destroyed, but it can be changed from one form to another.
According to this law, some heat given to the system is used to change the internal energy while the rest is used in doing work by the system.
It can be represented mathematically as
| \ |
Where,
- Q is the heat given or lost
- U is the change in internal energy
- W is the work done
We can also represent the above equation as follows,
| \ |
So we can infer from the above equation that the quantity is independent of the path taken to change the state. Further, we can say that internal energy tends to increase when the heat is given to the system and vice versa.
Process Of Transfer Of Matter Between An Open System And Its Surroundings
A system connected to its surroundings only through contact by a single permeable wall, but otherwise isolated, is an open system. If it is initially in a state of contact equilibrium with a surrounding subsystem, a thermodynamic process of transfer of matter can be made to occur between them if the surrounding subsystem is subjected to some thermodynamic operation, for example, removal of a partition between it and some further surrounding subsystem. The removal of the partition in the surroundings initiates a process of exchange between the system and its contiguous surrounding subsystem.
An example is evaporation. One may consider an open system consisting of a collection of liquid, enclosed except where it is allowed to evaporate into or to receive condensate from its vapor above it, which may be considered as its contiguous surrounding subsystem, and subject to control of its volume and temperature.
Also Check: Holt Mcdougal Larson Geometry Practice Workbook Answers
Entropy And Phase Space
Entropy is a very important thing in the realm of thermodynamics. Its the core idea behind the second and third laws and shows up all over the place. Essentially entropy is the measure of disorder and randomness in a system. Here are 2 examples
- Lets say you have a container of gas molecules. If all the molecules are in one corner then this would be a low entropy state . As the particle move out and fill up the rest of the container then the entropy increases.
- If you have a ball flying through the air then it will start off with its energy organised i.e. the kinetic energy of motion. As it moves through the air however, some of the kinetic energy is distributed to the air particles so the total entropy of system has increased
To get a more detailed picture of entropy we need to look at the concept of Phase Space. Some of the concepts for this may be a bit confusing but bear with me, once youve got your head round it its not that bad.
A phase space is just like a graph, but a point on this graph represents the whole state of a system. Lets use an example. Imagine I have a box with 4 gas particles inside. Each point in the phase space for this system tells you where all 4 balls are located in the box.
In all the diagrams I will depict the phase space as 2D to make it easier to convey what it actually represents. For our purposes we will not need to consider the dimensions.
Entropy can also be defined as the change when energy is transfered at a constant temperature
First Law Of Thermodynamics And Law Of Conservation Of Energy
Human metabolism also provides an example of energy conservation. human beings and other animals do work when they walk, run, or move heavy objects. work requires energy. energy is also needed for growth to make new cells and to replace old cells that have died. energy transforming processes that occur within organisms are named as metabolism. we can apply the first law of thermodynamics as : U = Q-Wto an organism of the human body. work W done will result in a decrease in the internal energy of the body. consequently the body temperature or in other words internal energy is maintained by the food we eat.
Also Check: Michael Jacksons Biological Son
The Next Step: One Way Street
So far, everything here is completely reversible. Whichever way you choose to express the First Law, you could stick minus signs on both sides of the equation. That means that if you had all the energy transfers go in the reverse direction, this wouldn’t violate the First Law at all. For example, one of the examples discussed above is that you have cellular respiration going on in your cells , and there is heat transfer from your body to the outside. Could this happen backwards? Can you take a heating pad, use it to transfer heat to your skin, and thereby make respiration run backwards, so that you end up with more chemical energy than you started with? Can you take in energy through heat and store this energy for later? The First Law doesn’t say no! Based on conservation of energy alone, there is no reason why this shouldn’t work.
Of course it doesn’t work. But to explain why, we’ll need the Second Law of Thermodynamics, and to understand the Second Law, we’ll need to use again our concepts about probability and randomness.
Ben Dreyfus 11/8/11, Wolfgang Losert 12/3/12
State And Explain The First Law Of Thermodynamics
Answer:
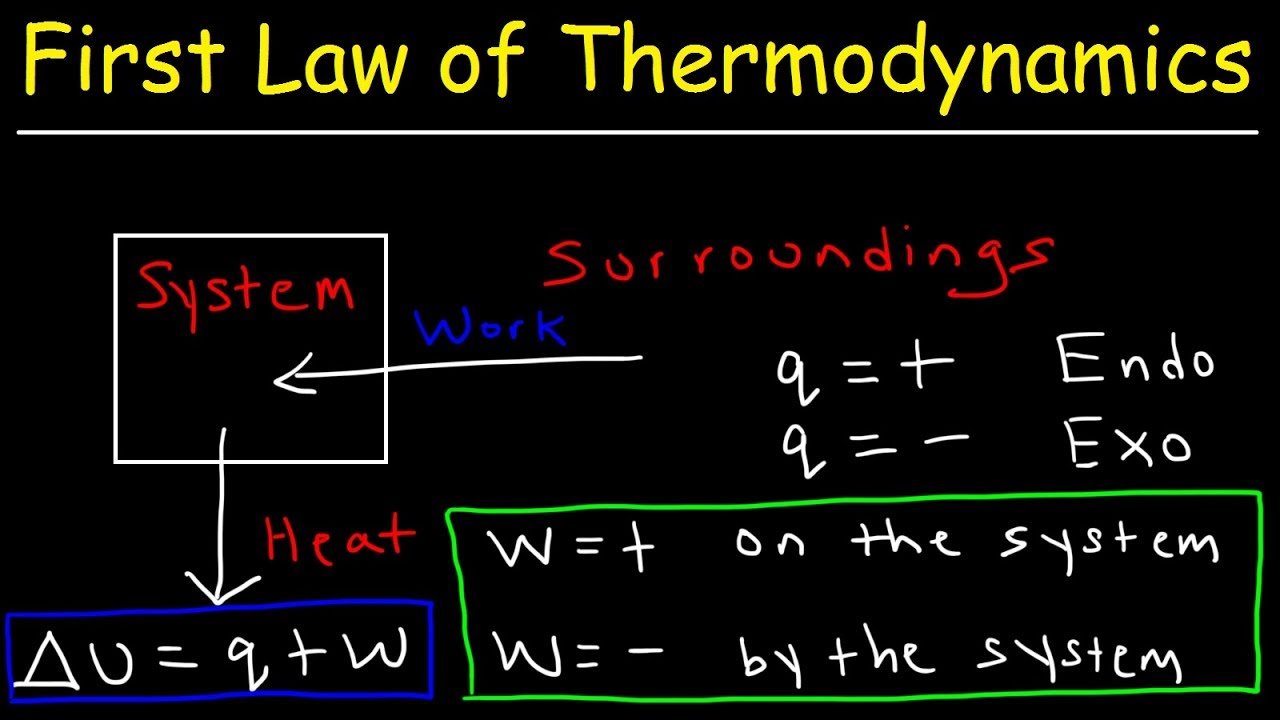
The First Law of Thermodynamics states that heat is a form of energy, and thermodynamic processes are therefore subject to the principle of conservation of energy. This means that heat energy cannot be created or destroyed. It can, however, be transferred from one location to another and converted to and from other forms of energy.
The mathematical representation of the first law of thermodynamics is:
| U = Q + W |
- U is the total change in the internal energy of the system
- Q is the heat exchanged between the system and its surroundings
- W is the work done either by the system or on the system
Below is the table explaining the sign conventions for heat, internal energy, and work done:
| U | ||
| It will be positive if the temperature increases | It will be positive if heat enters the gas | It will be positive if the gas is compressed |
| It will be negative if the temperature decreases | It will be negative if heat leaves the gas | It will be negative if the gas expands |
| It will be zero if the temperature is constant | It will be zero if no heat is exchanged | It will be zero if the volume is constant |
Don’t Miss: Lewis Structures And Molecular Geometry Models Of Covalent Bonding Lab Answers
Dropping The Macro Motion
But that’s not all! Sometimes you’ll see the First Law written as
$$U = Q + W$$
$$U = Q – W$$
where $U$ is only the internal energy, the energy associated with what’s happening inside the system. So $U$ includes the chemical and thermal energy of the molecules inside a system, but not the kinetic or potential energies associated with the motion and position of the system as a whole. So these representations of the First Law are valid only if the overall kinetic and potential energy of the system are not changing , and therefore any transfer of energy into or out of the system results in changes to internal energy.
The change in internal energy is literally the change in the sum of all the ½mv2’s of all the molecules plus their potential energies of binding into solids or liquids. While we can’t actually measure velocities and interaction potentials for every single molecule and interaction at the microscale, we can say something about the average energy associated with each motion and interaction: it will be proportional to the temperature!
So we can actually observe changes in internal motion simply by measuring changes in temperature.
Other Laws Of Thermodynamics
The first law of thermodynamics is arguably the most practically useful for a physicist, but the other three major laws are worth a brief mention too . The zeroth law of thermodynamics states that if system A is in thermal equilibrium with system B, and system B is in equilibrium with system C, then system A is in equilibrium with system C.
The second law of thermodynamics states that the entropy of any closed system tends to increase.
Finally, the third law of thermodynamics states that the entropy of a system approaches a constant value as the temperature approaches absolute zero.
Related Articles
Recommended Reading: How To Intimidate Someone Psychologically
Why Are The First And Second Laws Of Thermodynamics Important
The laws of physics explain how natural phenomena and machines work. These explanations not only satisfy our curiosity but also allow us to predict phenomena. In fact, they are instrumental in allowing us to build functional machinery.
As a branch of physics, thermodynamics is no exception for this. If you know how much energy in a system can be used for work, and how much will turn into heat , you can predict how much heat a given machine will produce under different conditions. Then, you can decide what to do with that heat.
Heat is a form of energy and if you know that energy cant be destroyed but only transformed, you could find a way to turn that thermal energy into mechanical energy which is what, in fact, heat engines do.
Given this basic application of the first and second laws of thermodynamics, you can probably imagine how useful they can be in the engineering field. But they can also have applications in chemistry, cosmology , atmospheric sciences, biology , and many other fields. Hence the importance of thermodynamics
An Example Of Work Done
Consider a gas in a cylinder at room temperature , with a volume of 0.065 m3. The gas is confined by a piston with a weight of 100 N and an area of 0.65 m2. The pressure above the piston is atmospheric pressure.
What is the pressure of the gas?
This can be determined from a free-body diagram of the piston. The weight of the piston acts down, and the atmosphere exerts a downward force as well, coming from force = pressure x area. These two forces are balanced by the upward force coming from the gas pressure. The piston is in equilibrium, so the forces balance. Therefore:
Solving for the pressure of the gas gives:
The pressure in the gas isn’t much bigger than atmospheric pressure, just enough to support the weight of the piston.
The gas is heated, expanding it and moving the piston up. If the volume occupied by the gas doubles, how much work has the gas done?
An assumption to make here is that the pressure is constant. Once the gas has expanded, the pressure will certainly be the same as before because the same free-body diagram applies. As long as the expansion takes place slowly, it is reasonable to assume that the pressure is constant.
If the volume has doubled, then, and the pressure has remained the same, the ideal gas law tells us that the temperature must have doubled too.
The work done by the gas can be determined by working out the force applied by the gas and calculating the distance. However, the force applied by the gas is the pressure times the area, so:
Don’t Miss: How Long Is The Ap Physics Exam