What Is Radioactivity
Radioactivedecay, or radioactivity, is basically a process in which an unstable nucleusloses energy by the emission of radiation in the form of a particle. Thisconcept is clearly explained in the following video:
This is more of a physics concept than a basic chemistry concept, but it is very relevant for chemists.
Not all atoms that exist are stable, some are what you could call not meant to be. Most of the matter that we see is made up of combinations of protons, neutrons that are stable . But other combinations of neutrons and protons give rise to unstable nuclei, that eventually fall apart. This is the basis of radioactivity in simple terms.
During the decomposition of these unstable atoms, energy is released in the form of particles. This release of energy can be detected, and it is what we call radiation. When this process takes place, a new nucleus is formed, and therefore, a new atom. This new atom can be also unstable and keep releasing radiation until it turns into an stable atom, which no longer emits energy as radiation.
Finally, I want to make an update with a 16th concept! This deserved a separate post, and here it is: A tutorial review on why chemicals react! If you are serious about learning chemistry, you need to master the basics behind the concepts of thermodynamics and kinetics.
If you are using this article to learn or teach high school chemistry, we have reviewed now the best books to learn chemistry at that level. You can check it out here.
Structure Of The Atom
An atom is the smallest unit of an element. There are 3 parts of an atom:
- Proton: positive electrical charge, found in the nucleus of an atom
- Neutron: neutral or no electrical charge, found in the nucleus of an atom
- Electron: negative electrical charge, found circling the nucleus
The size of the proton and neutron are similar, while the size of the electron is much, much smaller. The electrical charge of the proton and electron are exactly equal to each other, just opposite to each other. The proton and electron attract each other. Neither the proton nor the electron is attracted or repelled by the neutron.
Why Is Chemistry Important
Everythingis chemistry. Everything that you can observe macroscopically is made ofchemicals. You are made of chemicals, your food is chemicals, you breathechemicals, we live out of and thanks to chemicals, everything you see under thesun is a mixture of chemicals.
Everything you see or do is based on chemical concepts and processes. Fireworks going off takes place thanks to our understanding and application of chemistry. A medicine that you take to cure your illness does it so through chemical processes. A building doesnt fall off because we know chemistry. Obviously, all these examples result from a bunch of different branches of science coming together. Chemistry, as the central science, is in charge of gluing them together.
Also Check: What Is Sin In Geometry
Why Do Two Chemical Compounds React
Chemistrystudies changes in matter. A chemicalreaction is a process in which one set of chemical compounds aretransformed into another. Reaction occur when there is an interaction betweenthe compounds in which some initial bonds are broken and some new bonds areformed.
Why does this happen? In simple terms, because the energy holding the new bonds together is higher than the energy that held the initial bonds. This is the definition of a thermodynamically favored process. Favorable thermodynamics is the most fundamental step that leads two compounds to react with each other. Other drastically important factor is reaction kinetics.
What Is Chirality And Where Does It Come From
Chirality is a geometric property of certain molecules. A molecule is said to be chiral when its mirror image is not superimposable to the molecule itself. The classical source of chirality in a compound is the presence of a carbon atom with four different substituents. The concept is far better explained by this basic chemistry youtube video:
The origin of chirality, together with the origin of life is one of the most relevant questions in, not only chemistry, but science in general, so it is not possible to answer in a straightforward manner. The main theory backing it up is based on homochirality, which could have emerged over three steps: mirror-symmetry breaking, chiral amplification and chiral transmission. This is far beyond basic chemistry concepts, but you can further read about it here.
Also Check: What Is Interposition In Psychology
The Variable Density Of Water
| The density of water at constant pressure |
|---|
| Temp |
| 983.854 |
| The values below 0ºC refer to super cooled water |
Layers of water in a winter lake: During the winter months of seasonal climates, the warmest water in most lakes and rivers is only 4°C. This 4°C water has the highest density and sinks to the bottom of the lake. As the water becomes colder , it becomes less dense and rises to form ice on the surface of the lake. As a result, liquid water always exists in lakes and rivers during the winter months. This unique property of water enables animals and plants to survive under the frozen lake or winter, ensuring that all freshwater life does not go extinct each winter.
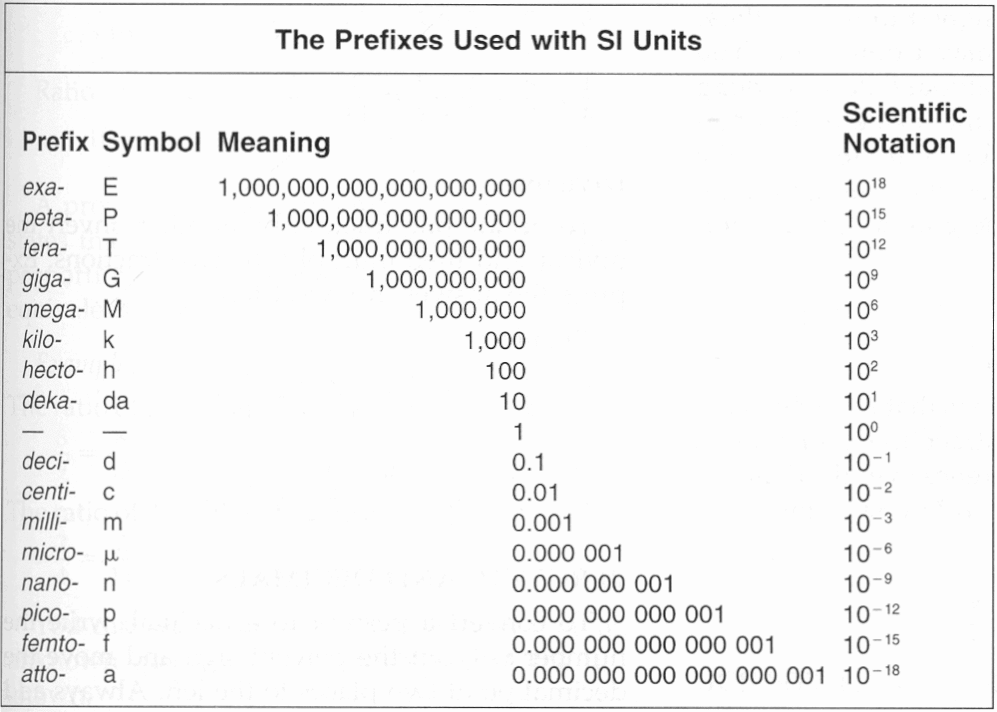
Section : Units Of Measurement
International System of Units and the Metric System
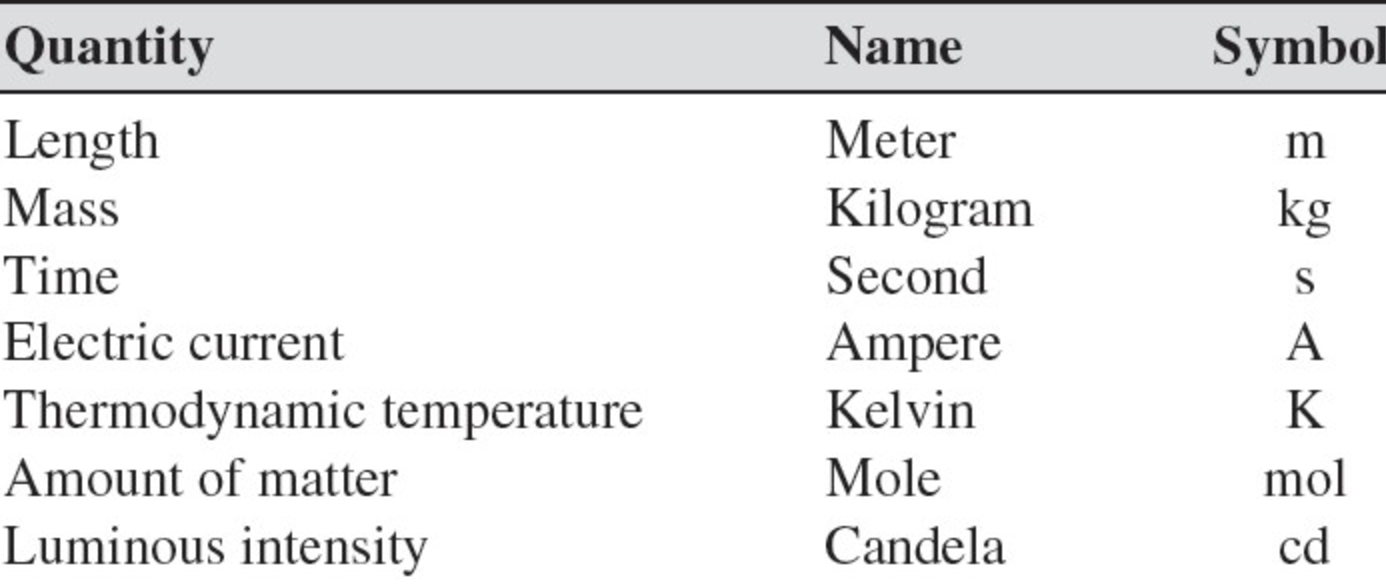
The International System of Units, abbreviated SI from the French Système International Dunités, is the main system of measurement units used in science. Since the 1960s, the International System of Units has been internationally agreed upon as the standard metric system. The SI base units are based on physical standards. The definitions of the SI base units have been and continue to be modified and new base units added as advancements in science are made. Each SI base unit except the kilogram is described by stable properties of the universe.
There are seven base units, which are listed in Table 1.2. Chemistry primarily uses five of the base units: the mole for amount, the kilogram for mass, the meter for length, the second for time, and the kelvin for temperature. The degree Celsius is also commonly used for temperature. The numerical relationship between kelvins and degrees Celsius is as follows
Read Also: How Did Greece’s Physical Geography Influence Its Early Civilizations
The History Of Measuring Temperature
- Isaac Newton proposed a thermometer with a scale of 12 degrees between the freezing and boiling points of water.
- Fahrenheit was working with tubes filled with mercury, which has a very high coefficient of thermal expansion. This, combined with the quality and accuracy of Fahrenheit’s work, led to much greater sensitivity, and his thermometer was standardized against a brine solution and universally adopted, with the Fahrenheit scale being named in his honor.
- Anders Celsius proposed a 100 degree scale for the difference between freezing and boiling of water, and after a few minor adjustments, the Celsius, or centigrade, system was also widely adopted.
Thermometer calibrated with the Celsius scal: Celsius is a scale and unit of measurement for temperature where 0 °C is the freezing point of water. Our ability to accurately measure temperature enables us to measure the weather, cook food accurately, or conduct a scientific experiment.
Key Takeaways: How To Learn Chemistry
- It’s possible to learn the basic concepts of chemistry online.
- Chemistry concepts should be studied in a logical order because concepts build upon each other. Jumping into the middle of the science can lead to confusion.
- While it’s fine to learn chemistry principles online, be aware that the lab component is an important part of the science. It’s a good idea to supplement textbook learning with experiments using a chemistry kit.
Don’t Miss: What Is Uncertainty In Chemistry
What Does Chemistry Mean
As an introduction to chemistry, it is the branch of science that studies matter and change. First, chemistry deals with the study of the composition and the properties of matter . Then, chemistry deals with change, or how these substances evolve when submitted to certain conditions, or how one substance changes or reacts while interacting with a different substance. The definition of chemistry cant be made shorter, since it covers basically everything!
What Is The Future Of Chemistry
Chemistryis the science that studies and manipulates matter. We human beings are gettingpretty good at it, , but we arefar from an ideal position in which we can easily make any molecule or compoundat will in a matter of minutes.
That isprobably the future of chemical synthesis, being able to shape any compound atwill in a matter of minutes, without relying on long term challenging synthesis projects. Furthermore, the possibilities ofsynthetic chemistry are literally endless: there will always be room for makinga chemical even better, or finding a molecule that works even better for agiven task.
Another keyaspect of the chemistry of the future will be reaching true fullsustainability. Chemistry will be one of the main branches of science to solvethe problem of energy.
Also,chemistry, as the central science, will be responsible to helping technologyand interdisciplinary science in general to develop smoothly.
Recommended Reading: Algebra With Pizzazz Did You Hear About Page 103
What Is The Simplest Structural Unit Of An Element Or Compound
From “Smarter than a 5th Grader”:
What is the simplest structural unit of an element or compound?
- molecule,
- neutron,
- proton
For covalent compounds, the answer would definitely be molecule. But I’m unclear as to how to show that the answer for ionic compounds or elements is “molecule”.
For example, states that ionic compounds: “Do not have molecules as basic structural unit. Instead extended array of positively and negatively charged particles called ions.” Is the term ‘structural unit’ defined across Science as only for covalent compounds?
An Example Of Chemistry Is The Feeling Of Affection And Attraction Between A Couple
. Understanding these atomic subparticles is important in understanding chemistry. Silicates are the minerals containing silicon and oxygen in tetrahedral SiO 4 4- units which are linked together in several patterns. An atom is defined as the basic unit of a chemical element.
10 Is the basic unit of life. There are seven base units which are listed in Table 12. A unit of measurement does not ever change and is based on a standard such as the SI standard meter kilogram etc used throughout the world or Engineering Units inch pound etc used in the USA.
An atom is the basic unit of matter with a nucleus at the center surrounded by negatively charged electrons E that move around the nucleus in orbits. 9 Is there a smallest unit of matter. A mole is simply a unit of measurement.
Chemistry is called the science of atoms and molecule Branches of Chemistry Organic Chemistry -This branch deals with study of carbon compounds especially hydrocarbons and their derivatives. Roald Hoffmann Science can be viewed as a continuing human effort to systematise knowledge for describing and understanding. Atoms Elements and the Nucleus.
The chemistry of life. It is the science not so much of the one hundred elements but of the infinite variety of molecules that may be built from them. The metric system is used because all metric units are based on multiples of 10 making conversions very.
The basic structural unit of silicates is SiO 4-4. SI Base Units.
Also Check: What Is Math Analysis In High School
Chapter 1 What Is Chemistry
If you are reading these words, you are likely starting a chemistry course. Get ready for a fantastic journey through a world of wonder, delight, and knowledge. One of the themes of this book is chemistry is everywhere, and indeed it is you would not be alive if it werent for chemistry because your body is a big chemical machine. If you dont believe it, dont worry. Every chapter in this book contains examples that will show you how chemistry is, in fact, everywhere. So enjoy the rideand enjoy chemistry.
What is chemistry? Simply put, chemistry is the study of the interactions of matter with other matter and with energy. This seems straightforward enough. However, the definition of chemistry includes a wide range of topics that must be understood to gain a mastery of the topic or even take additional courses in chemistry. In this book, we will lay the foundations of chemistry in a topic-by-topic fashion to provide you with the background you need to successfully understand chemistry.
What Is The Periodic Table
The periodic table is a list or arrangement of all known chemical elements. These are organized in a way that it allows grouping elements with similar atomic structure, and therefore, similar properties. The main criteria for this order is the arrangement by increasing atomic number, which is the number of particles of that elements nucleus . Its invention is attributed to the Russian chemist Dimitri Mendeleev, and in 2019 we celebrate the 150 anniversary of his original report in 1869.
Don’t Miss: Geometry Dash Practice Song Hack
What Is Oxidation And Reduction In Chemistry
Redox processes are a type of chemical reaction inwhich one of the reacting compounds gets oxidized and the other gets reduced. Aredox reaction involves a transfer of electrons. We say that a compound, oratom within a compound, gets oxidized when it loses electrons and the other component gets reduced when it gains electrons.
One of themost typical examples of a redox process is the rusting of iron. Iron metal, Fe0 reacts with oxygen from air, O2 to give rust, or iron oxide, Fe2O3.
4 Fe0 + 3 O2 2 Fe2O3
In this newcompound, the new oxidation state of iron is +3. Iron has lost 3 electrons,therefore, getting oxidized:
Fe0 Fe3+ + 3 e
On theother hand, the new oxidation state of oxygen is -2. Each oxygen atom hasgained two electrons, getting reduced:
O2 + 4 e 2 O2-
A typical exampleof a redox process is an explosion in which the explosive compound gets oxidizedviolently. C4 is a common plastic explosive, much more energetic than dynamite.The main component of C4 is RDX , also known ascyclonite or, according to IUPAC, 1,3,5-trinitro-1,3,5-triazinane.
The processof oxidation of RDX is thermodynamically favorable, and gives rise to aexothermic reaction, in which a large amount of energy is released in the formof heat and light, causing the explosion.
The NNO2 bonds are extremely unstable and prone to oxidation. Thats what makes this kind of compounds highly explosive.
Section : Chemistry And Matter
What is Chemistry?
Everything around us is made up of chemicals. From the color that makes a rose so red to the gasoline that fills our cars and the silicon chips that power our computers and cell phonesChemistry is everywhere! Understanding how chemical molecules form and interact to create complex structures enables us to harness the power of chemistry and use it, just like a toolbox, to create many of the modern advances that we see today. This includes advances in medicine, communication, transportation, building infrastructure, food science and agriculture, and nearly every other technical field that you can imagine.
Chemistry is one branch of science. Science is the process by which we learn about the natural universe by observing, testing, and then generating models that explain our observations. is the process by which we learn about the natural universe by observing, testing, and then generating models that explain our observations. Because the physical universe is so vast, there are many different branches of science . Thus, chemistry is the study of matter, biology is the study of living things, and geology is the study of rocks and the earth. Mathematics is the language of science, and we will use it to communicate some of the ideas of chemistry.
Figure 1.1: The Relationships Between Some of the Major Branches of Science.Chemistry lies more or less in the middle, which emphasizes its importance to many branches of science.
Physical vs. Chemical Properties
Recommended Reading: City Park Project For Geometry
What Are Acids And Bases
Following the original definition by Arrhenius, , an acid is a compound that is able to release a hydrogen cation, or proton . For example, molecules of hydrochloric acid get ionized in solution giving a proton to water, through an acid-base equilibrium:
HCl + H2O H3O+ + Cl
HCl in water gives rise to hydronium cations and chloride anions. This is classical acid-base equilibrium.
On the other hand, bases, such as sodium hydroxide , can catch protons from water, giving rise to hydroxide anions.
NaOH +H2O HO + Na+
Whereas the Arrhenius acid-base model is very illustrative, it has its limitations, and other models are used to describe more advanced acid-base theories. The most important ones are the Brønsted-Lowry theory , and the Lewis theory.
Accordingto the Lewis theory, an acid is asubstance that accepts a lone pair of electrons, and a base is a substance thatdonates a lone pair of electrons. This accounts for acid-base equilibria whichcannot be explained by Arrhenius or Brønsted-Lowry theories, such as thebasicity of ammonia in water:
:NH3 + H2O ) NH4+ +:OH
Relative acidity or basicity of solutions or mixtures is measured using a logarithmic scale called the pH scale. It generally goes from 0, most acidic , through pH = 7 , up to 14, most basic . Nevertheless, compounds more basic and acidic than those do exist, pH = 014 is definitely not a closed range. Examples of common solutions or mixtures of different pH are shown in the scale below.
What Is The Basic Unit Of Matter
An atom is the basic unit of matter. The atom is the basic building block of an element, and cannot be broken down further using any chemical means. An atom is made up of three particles: protons, neutrons and electrons.
The three particles of an atom carry different electrical charges. Protons carry a positive charge, electrons carry a negative charge, and neutrons have no electrical charge. Matter is anything that has mass and occupies space. All physical objects consist of matter in the form of atoms. Matter can exist as solid, liquid, gas or plasma. Some substances can transition between different states of matter depending on the amount of heat absorbed by the material.
Recommended Reading: What’s Osmosis In Biology