Molar Mass Of Ammonia Nh3 Step :
The first step for calculating molar mass is to identify all the elements in a given molecule and write their atomic masses using the periodic table. The atomic mass is equal to the atomic number which is listed below the element symbol. For example, if we are trying to find the molar mass of ammonia , then we need to find the atomic masses for nitrogen and hydrogen. Using the periodic table, we should get:
- Nitrogen: 14.01
- Hydrogen: 1.01
Worked Example Of Using Mole Ratio To Calculate Mass Of Reactant Or Product
The Question: 12.2 g of magnesium metal ) reacts completely with oxygen gas ) to produce magnesium oxide ).
Calculate the mass of oxygen consumed during the reaction and the mass of magnesium oxide produced.
How to Answer the Question:
Write the balanced chemical equation for the chemical reaction:
2Mg + O2 2MgO
Determine the mole ratio from the equation, Mg : O2 : MgO
moles : moles : moles is 2:1:2
Use the mole ratios to calculate the mass of O2 consumed and MgO produced as shown below:
- mass O2 = moles × molar mass
Calculate moles = mass ÷ molar mass
moles = 12.2 ÷ 24.31 = 0.50 mol
Use the balanced chemical equation to determine the mole ratio O2:Mg
moles : moles is 1:2
Solving For Mass Percent When Given Masses
Recommended Reading: Movement Definition Geography
Converting Between Mass Number Of Moles And Number Of Atoms
How many moles and how many atoms are contained in 10.0 g of nickel?
According to the periodic table, the atomic mass of nickel is 58.69 amu, which means that the molar mass of nickel is 58.69 g/mol. Therefore, we can divide 10.0 g of Ni by the molar mass of Ni to find the number of moles present.
Using dimensional analysis, it is possible to determine that:
10\text\times \frac}} = 0.170\text
To determine the number of atoms, convert the moles of Ni to atoms using Avogadros number:
0.170\text\times\frac \text}} = 1.02\times10^\text
Given a samples mass and number of moles in that sample, it is also possible to calculate the samples molecular mass by dividing the mass by the number of moles to calculate g/mol.
What is the molar mass of methane if there are 0.623 moles in a 10.0g sample?
\frac_4}_4} = 16.05 \text_4
The molar mass of CH4 is 16.05 g/mol.
Divide Change In Mass By Initial Mass
Finally, you divide the change in mass by the initial mass of your substance. This calculation shows what proportion of the initial mass changed.
To find the percent change, simply multiply this number by 100.
So 12 percent of the water in the beaker has evaporated over the course of your experiment. Note in your final answer whether the percent change is an increase or a decrease. If the initial mass is higher than the final mass, it is a decrease if final is higher than initial, it’s an increase.
You May Like: Michael Jackson Biological Children
Converting Atoms To Moles
Reversing the calculation above, it is possible to convert a number of atoms to a molar quantity by dividing it by Avogadros number:
\frac}} \frac}}}}= y\text
This can be written without a fraction in the denominator by multiplying the number of atoms by the reciprocal of Avogadros number:
x \text\cdot\frac}\text} = y \text
For example, if scientists know there are 3.5 \cdot 10^ atoms in a sample, they can calculate the number of moles this quantity represents:
3.5\times 10^\text\cdot\frac} \text} = 5.81\text
Chemical Computations With Avogadros Number And The Mole
Avogadros number is fundamental to understanding both the makeup of molecules and their interactions and combinations. For example, since one atom of oxygen will combine with two atoms of hydrogen to create one molecule of water , one mole of oxygen will combine with two moles of hydrogen to make one mole of H2O.
Another property of Avogadros number is that the mass of one mole of a substance is equal to that substances molecular weight. For example, the mean molecular weight of water is 18.015 atomic mass units , so one mole of water weight 18.015 grams. This property simplifies many chemical computations.
If you have 1.25 grams of a molecule with molecular weight of 134.1 g/mol, how many moles of that molecule do you have?
1.25\text \times \frac}}=0.0093 \text
The Mole, Avogadro: This video introduces counting by mass, the mole, and how it relates to atomic mass units and Avogadros number.
Read Also: What Percent Of The Mcat Is Physics
How Is A Mole Defined
A mole is defined as 6.02214076 × 1023 of some chemical unit, be it atoms, molecules, ions, or others. The mole is a convenient unit to use because of the great number of atoms, molecules, or others in any substance. The mole was originally defined as the number of atoms in 12 grams of carbon-12, but in 2018 the General Conference on Weights and Measures announced that effective May 20, 2019, the mole would be just 6.02214076 × 1023 of some chemical unit.
Examples Of Mass Percent Problems In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
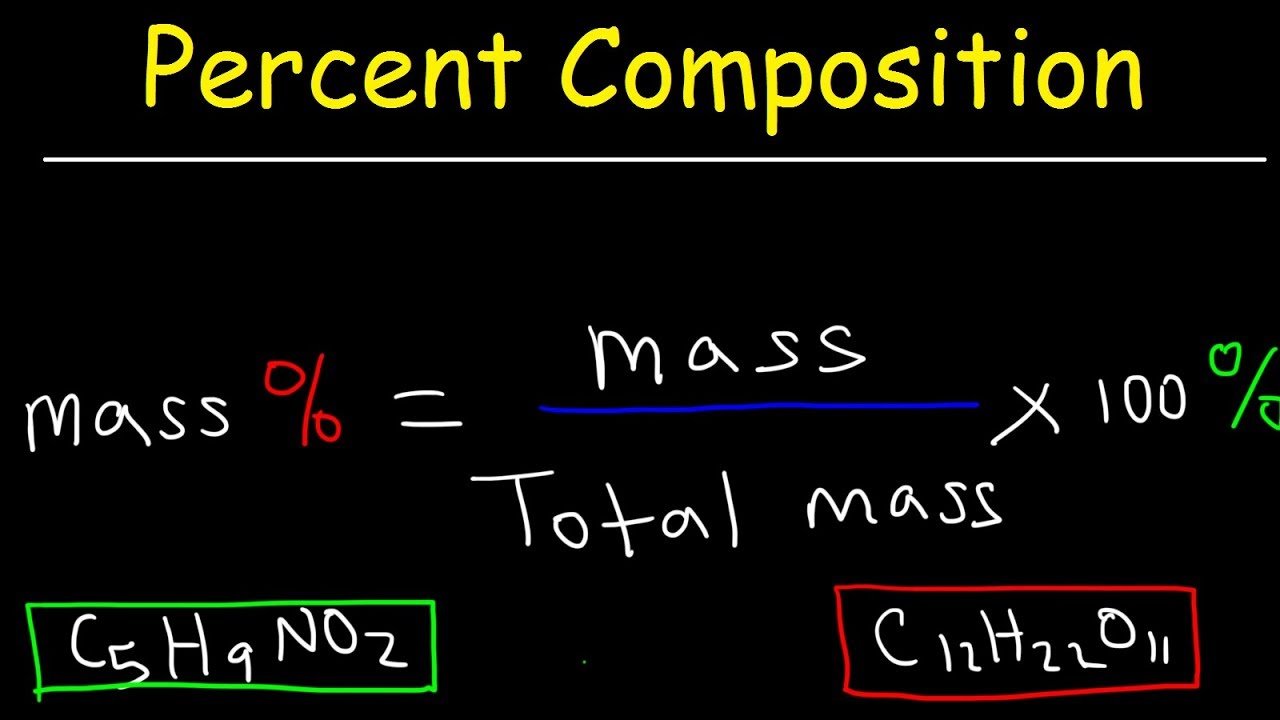
This is a worked example problem showing how to calculate mass percent composition. Percent composition indicates the relative amounts of each element in a compound. For each element, the mass percent formula is:
% mass = / x 100%
mass percent = x 100%
The units of mass are typically grams. Mass percent is also known as percent by weight or w/w%. The molar mass is the sum of the masses of all the atoms in one mole of the compound. The sum of all the mass percentages should add up to 100%. Watch for rounding errors in the last significant figure to make sure all the percentages add up.
Also Check: Algebra Nation Section 8 Answers
What Is Molar Mass
The definition of molar mass is simply the number of grams that one mole of a substance weighs. Another definition of molar mass, is the sum of the atomic weights of the atoms that make up a molecule. Both definitions will give you the same result, when calculating the molar mass of a molecule.
A mole of a substance is defined as 6.022 x 1023 atoms or molecules of that substance. 1023 is a one with 23 zeroes after it. Thats a heck of a lot of molecules, which is why the molar mass of table salt is a respectable 58.44 grams per mole, quite a large handful.
The units of molar mass is grams per mole, often abbreviated g/mol.
Formula Mass For Ionic Compounds
Ionic compounds are composed of discrete cations and anions combined in ratios to yield electrically neutral bulk matter. The formula mass for an ionic compound is calculated in the same way as the formula mass for covalent compounds: by summing the average atomic masses of all the atoms in the compounds formula. Keep in mind, however, that the formula for an ionic compound does not represent the composition of a discrete molecule, so it may not correctly be referred to as the molecular mass.
As an example, consider sodium chloride, NaCl, the chemical name for common table salt. Sodium chloride is an ionic compound composed of sodium cations, Na+, and chloride anions, Cl, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu .
Figure 3.
Recommended Reading: Segment And Angle Addition Postulate Worksheet
Worked Examples Of Calculating Mass Moles Molar Mass
In each of the worked examples below, you will be asked to calculate either the moles, mass, or molar mass of a pure substance.
To answer each question correctly you will need to:
Tips For Success Calculating Mass Percent
- You won’t always be given the total mass of a mixture or solution. Often, you’ll need to add up the masses. This might not be obvious. You may be given mole fractions or moles and then need to convert to a mass unit.
- Watch your significant figures.
- Always make sure the sum of the mass percentages of all components adds up to 100%. If it doesn’t, you need to go back and find your mistake.
Don’t Miss: Algebra 2 Domain And Range Worksheet Answer Key
Calculating The Mass Of Product
that can be made in a chemical reaction.
It can be calculated using:
- the relative formula mass of the product
Worked example 1
-
Carbon reacts with oxygen to produce carbon dioxide:
C + O2 CO2
Calculate the maximum mass of carbon dioxide that can be made from 6.0 g of carbon and an excessof oxygen.
- Reveal answer
-
relative formula mass, Mr, of CO2 = 12.0 + = 44.0
Looking at the balanced equation:
- sum of Ar for C = 12.0
- sum of Mr for CO2 = 44.0
= 22.0 g
-
Nitrogen reacts with hydrogen to produce ammonia:
N2 + 3H2 2NH3
Calculate the maximum mass of ammonia that can be made from an excess of nitrogen and 12.0 g ofhydrogen.
- Reveal answer
-
relative formula mass, Mr, of H2 = = 2.0
relative formula mass, Mr, of NH3 = 14.0 + = 17.0
- sum of Mr for H2 = = 6.0
- sum of Mr for NH3 = = 34.0
= 68.0 g
-
Lithium hydroxide is used to absorb exhaled carbon dioxide in spacecraft:
2LiOH + CO2 Li2CO3 + H2O
Calculate the maximum mass of water that can be made from an excess of carbon dioxide and 95.6 gof lithium hydroxide.
- Reveal answer
-
relative formula mass, Mr, of LiOH = 6.9 + 16.0 + 1.0 = 23.9
relative formula mass, Mr, of H2O = + 16.0 = 18.0
Looking at the balanced equation:
- sum of Mr for LiOH = = 47.8
- sum of Mr for H2O = 18.0
= 36.0 g
How To Calculate Molar Mass With Examples
Well go through three examples progressing from easy to difficult. By the end of this post, youll be able to calculate the molar mass of anything.
Example #1: Single element
Sodium
Finding the molar mass of a single element is really simple. All you need to do is find the atomic mass of the element on the periodic table and report the number with the unit grams per mole or g/mol.
From this, you can see that sodiums molar mass will be 22.99 g/mol.
Example #2: Simple compound
CH3OH
Start by determining how many of each elements there are by looking the subscripts . In this compound, there are 1 C, 4 H , and 1 O. Next, multiply the number of a particular element by its molar mass. Finally, add the products together and youll arrive at the answer.
Example #3: Complicated compound
2CO3
The process is very similar to calculating the molar mass of a simple compound. The only difference is youll need to multiply the subscript on the outside of the parenthesis by the subscripts inside the parenthesis.
Start by determining how many of each elements there are. In this compound, there 2N, 8H, 1C, and 3O. Next, multiply the number of a particular element by its molar mass. Finally, add the products together and youll arrive at the answer.
After reading and working through these three examples, you should be able to calculate the molar mass of anything.
Recommended Reading: What Is An Independent Variable Math
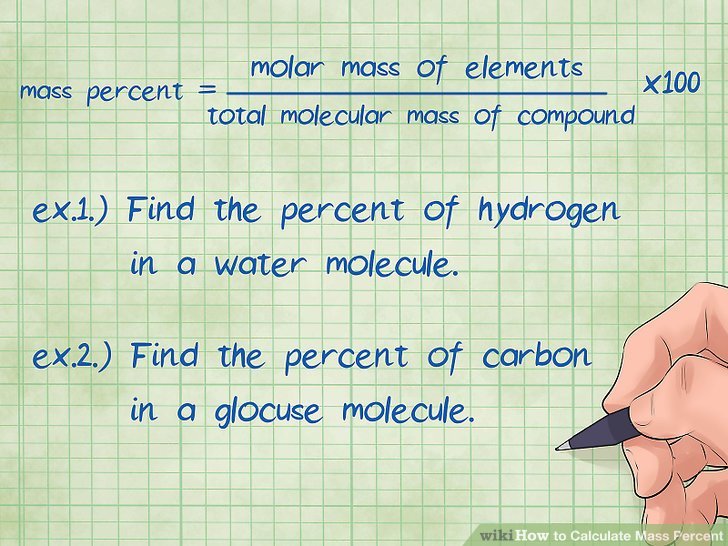
How To Calculate Mass Percent
This article was co-authored by Bess Ruff, MA. Bess Ruff is a Geography PhD student at Florida State University. She received her MA in Environmental Science and Management from the University of California, Santa Barbara in 2016. She has conducted survey work for marine spatial planning projects in the Caribbean and provided research support as a graduate fellow for the Sustainable Fisheries Group.wikiHow marks an article as reader-approved once it receives enough positive feedback. In this case, several readers have written to tell us that this article was helpful to them, earning it our reader-approved status. This article has been viewed 565,510 times.
Mass percent tells you the percentage of each element that makes up a chemical compound.XResearch source Finding the mass percent requires the molar mass of the elements in the compound in grams/mole or the number of grams used to make a solution.XResearch source It is simply calculated using a basic formula dividing the mass of the element by the mass of the compound .
Calculating The Moles Of A Pure Substance
In the discussion above, we discovered that we could calculate the mass of a pure substance using the moles and molar mass of the substance:
mass = moles × molar mass
How would we calculate the moles of pure substance if we knew the mass of the substance?
We could use some algebra: divide both sides of the equation by the molar mass:
| mass |
moles = mass ÷ molar mass
n = m ÷ M
Follow these steps to calculate the amount of pure substance in moles given the mass of substance:
Step 1. Extract the data from the question:
mass = m = write down what you are told in the question
moles = n = ?
molar mass = M = write down what you are told in the question
Step 2. Check the units for consistency and convert if necessary:
Mass must be in grams !
If mass is given in milligrams , divide it by 1,000 to give the mass in grams .
If mass is given in micrograms , divide it by 1,000,000 to give a mass in grams .
If mass is given in kilograms , multiply it by 1,000 to give a mass in grams .
Step 3. Write the mathematical equation :
moles = mass ÷ molar mass
n = m ÷ M
M = m ÷ n
or We could use some logic:
Therefore, molar mass = mass ÷ moles
or you can write
M = m ÷ n
Follow these steps to calculate the molar mass of a pure substance given the amount of substance in moles and the mass of substance:
Step 1. Extract the data from the question:
mass = m = write down what you are told in the question
moles = n = write down what you are told in the question
molar mass = M = ?
M = m ÷ n
Recommended Reading: Beth Thomas Psychopath
Unlock This Answer Now
Start your 48-hour free trial to unlock this answer and thousands more. Enjoy eNotes ad-free and cancel anytime.
Already a member? Log in here.
In order to do this calculation we first need to understand the concept of the mole. Just as a “dozen” of something means 12 items, or a “pair” means 2, in chemistry a “mole” of something means you have 6.02 x 10^23 items.
When you look at a periodic table of the elements, you see that sodium has an atomic weight of 23. This means that a single atom of sodium weighs 23 atomic mass units , but it also means that one mole of sodium atoms weighs 23 grams. Hence 6.02 x 10^23 atoms of sodium weight a total of 23 grams. This means that if we divide 23 grams by the number of atoms, we should have the weight of a single atom.
23 / 6.02 x 10^23 = 3.8 x 10^-23 grams
So one atom of sodium weighs 0.000000000000000000000038 grams
Further Reading
How Is A Mole Calculated
If you want to know how many moles of a material you have, divide the mass of the material by its molar mass. The molar mass of a substance is the mass in grams of one mole of that substance. This mass is given by the atomic weight of the chemical unit that makes up that substance in atomic mass units . For example, silver has an atomic weight of 107.8682 amu, so one mole of silver has a mass of 107.8682 grams.
mole, also spelled mol, in chemistry, a standard scientific unit for measuring large quantities of very small entities such as atoms, molecules, or other specified particles.
The mole designates an extremely large number of units, 6.02214076 × 1023. The General Conference on Weights and Measures defined the mole as this number for the International System of Units effective from May 20, 2019. The mole was previously defined as the number of atoms determined experimentally to be found in 12 grams of carbon-12. The number of units in a mole also bears the name Avogadros number, or Avogadros constant, in honour of the Italian physicist Amedeo Avogadro . Avogadro proposed that equal volumes of gases under the same conditions contain the same number of molecules, a hypothesis that proved useful in determining atomic and molecular weights and which led to the concept of the mole.
You May Like: Half Life Equation Chemistry