What Can Influence A Pesticide’s Vapor Pressure In The Environment
Many things can affect whether or not a pesticide will get into the air. In fact, pesticides can have different vaporpressures under different conditions. Temperature plays a very large role here.7 In general, pesticides will havelower vapor pressures at lower temperatures. As temperature goes up, so does the pesticide’s vapor pressure.
Other environmental factors, like climate, soil type, and moisture level can also affect a pesticide’s volatility.Pesticides can also stick to soil, be broken apart by water and light, and dissolve in water. These things can affecthow likely it is to become a vapor. The way a pesticide is applied can also play a role.7 For example, a pesticide withsmall droplets, like an aerosol, may get into the air more easily than a spray with larger droplets.
Additionally, the other ingredients in a pesticide product can speed up or slow down the active ingredient’stransition to the vapor phase.8 A quick example of how some of these factors work together can be found in thefollowing images.
Liquid Mixtures: Raoult’s Law
Raoult’s law gives an approximation to the vapor pressure of mixtures of liquids. It states that the activity of a single-phase mixture is equal to the mole-fraction-weighted sum of the components’ vapor pressures:
- P
^}} is the vapor pressure of component i . Raoult’s law is applicable only to non-electrolytes it is most appropriate for non-polar molecules with only weak intermolecular attractions .
Systems that have vapor pressures higher than indicated by the above formula are said to have positive deviations. Such a deviation suggests weaker intermolecular attraction than in the pure components, so that the molecules can be thought of as being “held in” the liquid phase less strongly than in the pure liquid. An example is the azeotrope of approximately 95% ethanol and water. Because the azeotrope’s vapor pressure is higher than predicted by Raoult’s law, it boils at a temperature below that of either pure component.
There are also systems with negative deviations that have vapor pressures that are lower than expected. Such a deviation is evidence for stronger intermolecular attraction between the constituents of the mixture than exists in the pure components. Thus, the molecules are “held in” the liquid more strongly when a second molecule is present. An example is a mixture of trichloromethane and 2-propanone , which boils above the boiling point of either pure component.
Finding Vapor Pressure With Dissolved Solutions
Recommended Reading: Why Am I Always Late Psychology
Interesting Applications Of Surface Tension & Vapor Pressure
Surface tension is what lets bugs skate across the surface of the water since their weight is less than the surface tension, they can glide without breaking the hydrogen bonds enough to sink through. Droplets are also a result of surface tension, droplets together counter to gravity!
The separation of different liquids is also achieved using vapor pressure. Some liquids will evaporate faster than others and as a result, the vapor will condense in a different concentration as the starting liquid. This is known as fractional distillation and is a common purification technique used in organic chemistry labs.
What Is Surface Tension
Surface tension is a measure of the intermolecular forces present at the surface of a liquid. An easy way to think about it is the amount of force that is required to break the surface of a liquid.
A liquid, such as water, with hydrogen bonding present will have high surface tension. Because hydrogen bonds are so strong, there will be a higher amount of force needed to break the bonds and distort the surface barrier of the liquid. On the other hand, liquids with weaker intermolecular forces, such as benzene, correspondingly have low surface tension. These types of liquids contain bonds that can be broken more easily, so the surface is more likely to be distorted.
Recommended Reading: How Do Noise Cancelling Headphones Work Physics
Cultural Definitions Forvapor Pressure
In physics and chemistry, the atmospheric pressure that would be exerted by any single component of a gas if that component were the only one present. For example, the vapor pressure of oxygen in the atmosphere of the Earth is the pressure that would exist if everything but oxygen were removed. The total atmospheric pressure is the sum of the vapor pressures of all the materials in the atmosphere.
Vapor Pressure Of Water
The vapor pressure of water is the pressure at which water vapor is in thermodynamic equilibrium with its condensed state. If we raise the pressure and keep the temperature, the water will condense.Have a look at this handy vapor pressure for water table to find the pressure for different temperatures quickly:
| T |
|---|
Read Also: G Definition Physics
What Is The Heat Of Vaporization
As we provide heat to a liquid, its energy increases, which results in an increase in the overall temperature. At the boiling point, the additional heat is used up by the molecules to overcome the intermolecular force of attraction in the liquid and change to the gaseous state.
When 1 mole of liquid is transformed into a gaseous state, the amount of heat provided by this process is known as the Heat of Vaporization.
Which Has Maximum Vapour Pressure
The material with the lowest boiling point would, therefore, have the highest vapour pressure at room temperature . The highest boiling point material will have the lowest vapour pressure. Vapour pressure is an evaporation-related fluid element.
This was just a quick intro about the vapour pressure. To find out more about different states of liquid, please download BYJUS the learning app.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: Ccl4 Bond Angles
Vapour Pressure Of Solutions Of Solids In Liquids
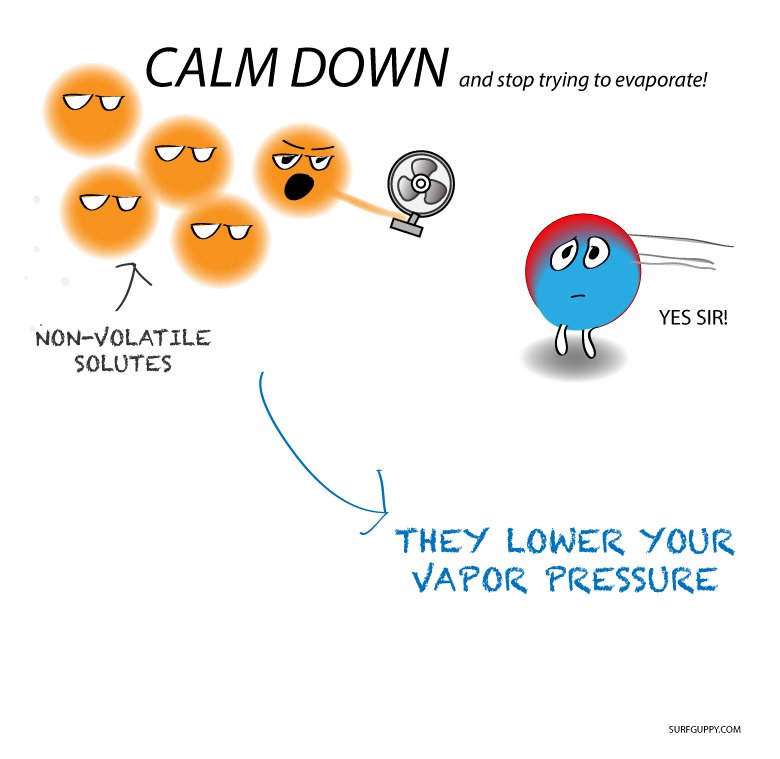
Let us now look at the other type of solutions i.e. solids in liquid solution. Here we consider a solid as the solute, while the solvent is a liquid. In these cases, the solute is non-volatile in nature. The vapour pressure is less than the pure vapor pressure of the solution. Let us now see how we can find the overall vapour pressure of such a solution.
We consider a solution in which A is the solvent and B is the solute. Applying the Raoults Law, we know that partial vapour pressure of individual component is directly proportional to its mole fraction.
Now, if we add a non-volatile solute, it is imperative that vapour pressure comes only from the solvent part. This is because they are the only available component in the vapour phase. Hence, if PA is the vapor pressure of the solvent, xA is its mole-fraction and PA0 is the vapor pressure of the pure solvent, then by Raoults law, the relation will come out to be:
PA xA
PA = PA0 xA
When we plot a graph between mole fraction of solvent and vapour pressure, we find its nature to be linear.
To Keep Your Risk Low In Your Own Home You May Consider:
- Covering vents when spraying nearby.
- Turning off air circulation to rooms being sprayed.
- Using volatile pesticides only in well-ventilated areas.
- Avoiding the use of volatile pesticides near exposed food items.
- Ventilating the area after application by opening windows and doors and/or using fans.
- Keeping people and pets out until the area has been well-ventilated.
- Avoiding treated areas until sprays have dried thoroughly.
Recommended Reading: Who Are The Biological Parents Of Prince Paris And Blanket
What Is The Differences Between Partial Pressure And Vapour Pressure
Was looking at Henry’s law and Raoult’s lawconstants and there seemto be lots ofequations involved.Henry’s law involves partial pressure and the latter involves the vapor pressure.Wondering what the difference is?
In a mixture of gases, each gas has a partial pressure which is the hypothetical pressure of that gas if it alone occupied the volume of the mixture at the same temperature.
What does this mean? For example, if we have a mixture of gases $A$, $B$ and $C$ in an isolated room, then, according to Dalton’s law, the pressure exerted by the gases will be the sum of their partial pressures :$$P = p_A + p_B + p_C$$where $p_A$, $p_B$ and $p_C$ are the partial pressures of each gas. Also, if we have a moles of $A$, b moles of $B$ and c moles of $C$, we can express the partial pressure of each gas as below:$$p_A = \frac P$$$$p_B = \frac P$$$$p_B = \frac P$$
Vapor pressure or equilibrium vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases at a given temperature in a closed system.
This may look just like Dalton’s law, but there are different pressures involved. The partial pressure of a gas is the pressure exerted by a gas in the volume occupied by a mixture of gases, while the vapor pressure of a gas is the pressure exerted by a gas over its condensed phase.Although :
The vapor pressure that a single component in a mixture contributes to the total pressure in the system is called partial pressure.
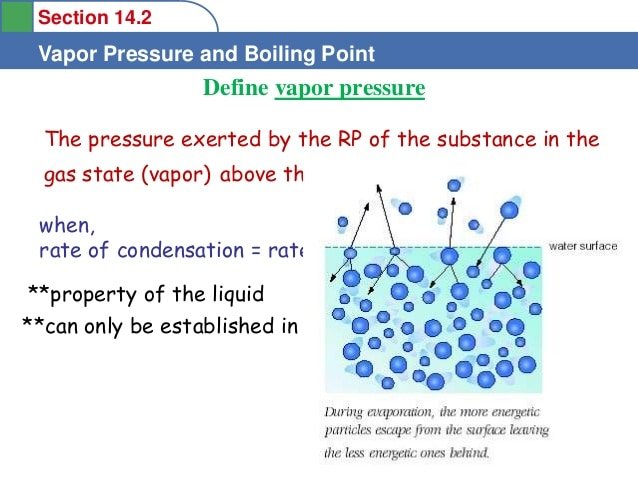
Characteristics Of Vapour Pressure
However, as time passes, the number of molecules in the vapour phase increases while the rate of condensation also increases. It reaches a stage where the rate of evaporation is equal to the rate of condensation. This phase is called the stage of equilibrium.
As represented by the manometer, at this point the pressure exerted by the molecules is called the vapour pressure of the liquid. Vapour pressure is defined as the pressure exerted by the vapour present above the liquid.
Temperature is the sole factor that affects vapour pressure. The vapour pressure of a liquid is independent of the volume of liquid in the container, whether one litre or thirty litres both samples will have the same vapour pressure at the same temperature. Temperature has an exponential connection with vapour pressure, which means that as the temperature rises, the vapour pressure rises as well.
The process of evaporation depends on different factors:-
Recommended Reading: What Is The Molecular Geometry Of Ccl4
Boiling Point Of Water
Like all liquids, water boils when its vapor pressure reaches its surrounding pressure. In nature, the atmospheric pressure is lower at higher elevations and water boils at a lower temperature. The boiling temperature of water for atmospheric pressures can be approximated by the Antoine equation:
- log
| 20 |
Finding Vapor Pressure In Special Cases
Also Check: Lesson 1.7 Practice A Geometry Answers
Pollutant Transport To The Atmosphere
Vapor pressure is the pressure exerted by a vapor in a confined space.5 The term vaporization is frequently used synonymously with vapor pressure, but it is actually the change of a liquid or solid to the vapor phase. Volatilization is a function of the concentration of a contaminant in solution and the contaminants partial pressure. That is, proportionality between solubility and vapor pressure can be established for any chemical. This means that volatilization is a common means by which an air pollutant reaches the atmosphere. Henry’s law expresses this proportionality, that is, it states that the concentration of a dissolved gas is directly proportional to the partial pressure of that gas above the solution :
where, CW is the concentration of gas in water.
A good way to estimate the likelihood of a chemical’s movement to the atmosphere is by combining the concentration of a dissolved contaminant and its partial pressure in the air just above where the chemical resides, that is, the headspace, when the concentrations in both locations are at equilibrium. In many situations, the headspace is actually the open atmosphere.
where, R is the gas constant and T is the temperature .
A number of databases provide physicochemical information, including partitioning coefficients. Some are provided in Appendix 1.
James G. Speight Ph.D., D.Sc., in, 2019
What Is This Pan Used For
Evaporation pan. Courtesy of NOAA.
Water loss to the atmosphere is a significant problem in many parts of the world. When water supplies are low, anything that can be done to decrease water loss is important for farmers. An evaporation pan can be used to measure how fast water evaporates in a given location. This information can be used as part of projects to develop ways to cut down on evaporation and increase the amount of usable water in a region.
Also Check: Ccl4 Resonance Structures
How Are Vapor Pressure And Boiling Point Related
The boiling point decreases as the vapour pressure increases.
Explanation:
Vapour Pressure
Some of the molecules at the surface of a liquid have enough kinetic energy to escape into the atmosphere.
These molecules exert a pressure on the walls of a closed container.
The vapour pressure is the pressure exerted when the molecules leave the surface at the same rate as they return.
If the intermolecular forces in a liquid are small, the molecules can easily escape from the surface of the liquid.
The liquid will have a high vapour pressure.
Boiling Point
The normal boiling point is the temperature in which the vapour pressure of a liquid becomes equal to atmospheric pressure.
If the intermolecular forces are small, the liquid has a high vapour pressure.
Little heat energy will have to be added to separate the molecules, so the boiling point will be low.
Conversely, if there are strong intermolecular forces, the molecules will be strongly attracted to each other.
Few molecules will enter the gas phase, and the vapour pressure will be low.
More heat will be required to separate the molecules, so the boiling point will be higher.
What Is Vapour Pressure In Chemistry
Vapour pressure is a measure of the tendency of a material to change into the gaseous or vapour state, and it increases with temperature. The temperature at which the vapour pressure at the surface of a liquid becomes equal to the pressure exerted by the surroundings is called the boiling point of the liquid.
Recommended Reading: Hawkes Learning Answers
Vapor Pressure Of A Solvent Is Bigger Than Vapor Pressure Of A Solution
Compound, like solution, has an entropy or energy bigger than single material or pure solvent. The entropy rising also rise the energy that is needed to transform solvent molecule from liquid phase to gas phase.
How to calculate the vapor pressure lowering of a solution? First, we emphasize the vapor pressure lowering occurs in a non-volatile solvent. Non-volatile is a property that makes a substance dies not evaporate easily while volatile means that a substance can evaporate easily.
The correlation between saturated solution vapor pressure with its solvent vapor pressure already been explained by Francois M. Raoult where he explained below formula:
P = Xp.Po
P = Saturated solution vapor pressurePo = Pure solvent vapor pressureXp = Solvent mole fraction
P = PoP
X water + X urea = 1
Xp + Xt = 1Xp = 1 Xt
P = PoP Po = Xt . Po