What Does Po4 Stand For
What does PO4 mean? This page is about the various possible meanings of the acronym, abbreviation, shorthand or slang term: PO4.
Filter by:
Couldn’t find the full form or full meaning of PO4?
Maybe you were looking for one of these abbreviations:
Discuss these PO4 abbreviations with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.
Better Ageing And Cycle
LFP chemistry offers a longer cycle life than other lithium-ion approaches.
LiFePO4 cells experience a slower rate of capacity loss than lithium-ion battery chemistries such as cobalt or manganese spinel lithium-ion polymer batteries or lithium-ion batteries. After one year on the shelf, a LiCoO2 cell typically has approximately the same energy density as a LiFePO4 cell, because of LFP’s slower decline of energy density.
Medical And Biological Research Uses
The medicinal type of phosphorus is phosphate. Some phosphates, which help cure many urinary tract infections, are used to make urine more acidic. To avoid the development of calcium stones in the urinary tract, some phosphates are used. For patients who are unable to get enough phosphorus in their daily diet, phosphates are used as dietary supplements, usually because of certain disorders or diseases. Injectable phosphates can only be handled by a health care provider.
Plants take up phosphorus through several pathways: the arbuscular mycorrhizal pathway and the direct uptake pathway.
Don’t Miss: How Old Is Elton Johns Kids
What Is The Chemical Formula For A Phosphate Ion
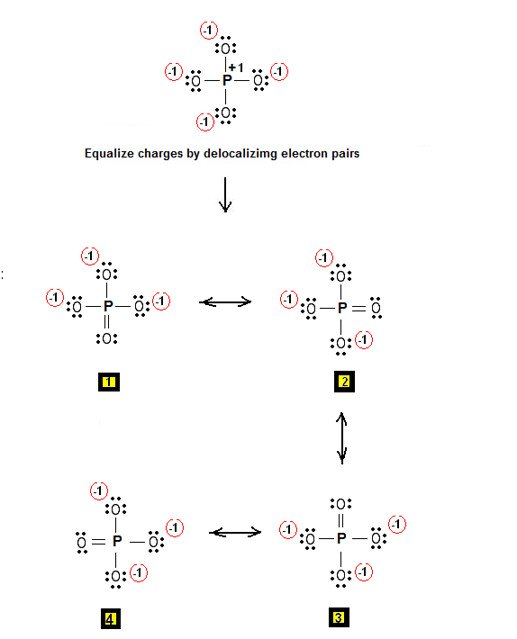
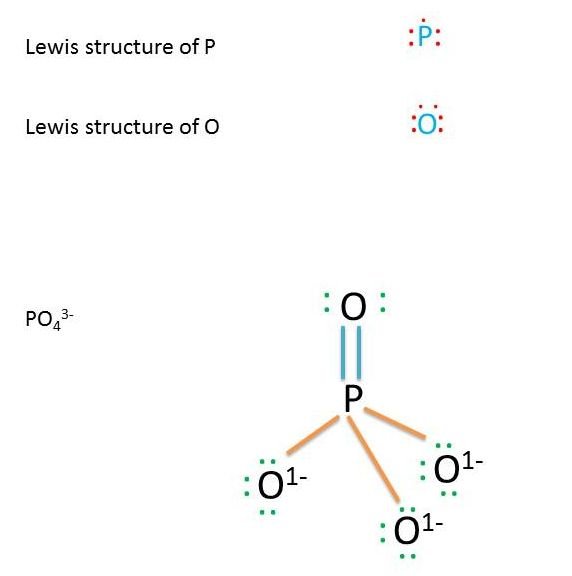
The chemical formula for a phosphate ion is 3-. The formula indicates that one ion of phosphate contains one atom of phosphorus, represented by the P and four atoms of oxygen, symbolized by O4. The superscript 3- indicates that the ion carries a charge of -3.
In aqueous form, phosphate is derived from phosphoric acid, which is represented by the formula H3PO4, when the acid loses three protons in the form of the three hydrogen ions. Inorganic phosphate can be formed by the reaction between pyrophosphate and water. Phosphate has a molar mass of 94.97 grams per mole. Natural phosphate can be found and mined from some types of rocks.
Preparation Of Calcium Phosphate
It can also be produced by reacting phosphoric acid with solid calcium hydroxide Ca2
Ca2+H3PO4Ca32+H2O
3Ca2+2H3PO4Ca32+6H2O
Dibasic calcium phosphate can be produced in the former of these reactions by using an aqueous solution of calcium hydroxide. Monobasic calcium phosphate is obtained by adding excess phosphoric acid to either a dibasic solution or a tribasic calcium phosphate solution and letting the solution to evaporate.
Don’t Miss: Holt Geometry Chapter 7 Test Form C Answers
High Peak Current/power Ratings
LiFePO
ion is stronger than the CoO bond in the ion, so that when abused ” rel=”nofollow”> overheated, etc.), the oxygen atoms are released more slowly. This stabilization of the redox energies also promotes faster ion migration.
As lithium migrates out of the cathode in a LiCoO2 cell, the CoO2 undergoes non-linear expansion that affects the structural integrity of the cell. The fully lithiated and unlithiated states of LiFePO4 are structurally similar which means that LiFePO4 cells are more structurally stable than LiCoO
No lithium remains in the cathode of a fully charged LiFePO4 cell. LiFePO4 is highly resilient during oxygen loss, which typically results in an exothermic reaction in other lithium cells. As a result, LiFePO4 cells are harder to ignite in the event of mishandling . The LiFePO4 battery does not at high temperatures.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
You May Like: Define Elastic Force
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Health Hazards Associated With Calcium Phosphate
When the toxic doses ingested become more than 2 g/kg unusual skin sensitization occurs. Inhaling it may cause chemical pneumonitis. Calcium phosphate is used in many products in biomedicine, but also in dentistry and cosmetics. In certain situations, it is found in nanoparticle form, either on purpose or after degradation or mechanical abrasion. Possible issues refer to the biological impact of these nanoparticles.
A thorough literature review shows that calcium phosphate nanoparticles, as such, do not have inherent toxicity, but may lead to an increase in intracellular calcium concentration following endosomal uptake and lysosomal degradation. However, cells are able to remove calcium from the cytoplasm within a few hours, unless very high doses of calcium phosphate are used.
The cytotoxicity observed in some cell culture studies , especially for unfunctionalized particles, is likely due to particle agglomeration and subsequent sedimentation on the cell layer, leading to a very high local concentration of particles, high absorption of particles, and subsequent cell death.
Calcium phosphate nanoparticles can reach the bloodstream by inhalation, but no harmful effects have been observed with the exception of extended exposure to high particle doses. Calcium phosphate nanoparticles within the body do not pose a risk because they are normally resorbed and destroyed by osteoclasts and macrophages.
Don’t Miss: Geometry Dash 1.9
How Is Phosphorus Used In Everyday Life
Phosphorus is a essential nutrient in plants and its main use through phosphate compounds is in fertilizer production. Just as there are biological cycles of carbon and nitrogen, there is a phosphorus cycle. Phosphorus is used for the production of safety matches , pyrotechnics, and fire shells.
Po4 Blood Test Results Explained
Medical providers will often order a PO4 blood test to measure phosphorus when other vitamins and minerals are being measured. The goal is to determine if there is a deficiency within the body that could be causing bothersome physical symptoms. It can also be used as part of the process to determine if there is a parathyroid issue which may need to be addressed. Most people will have the PO4 blood test ordered because there was an abnormal result on a previous calcium test.
Eating a meal before having this blood test can dramatically affect the results. Most medical providers will require overnight fasting when the PO4 blood test is ordered and there may be medication restrictions in place as well.
Read Also: 100 Day Countdown To The 5th Grade Math Fsa (1-50) Answer Key
Most People Have Abnormal Phosphorus Detected On An Annual Exam
Because most people wont even realize their phosphorus levels are out of balance, the most common way to discover the need for a PO4 blood test is through the annual basic metabolic panel or a comprehensive metabolic panel. These test calcium levels as part of the test and can lead medical providers toward the need for the PO4 blood test.
One common concern is that vegetarians may not be able to meet their phosphorus needs and some medical providers even suggest regular PO4 testing because of this dietary choice. About half of the phosphorus that is in vegetarian sources is absorbed by the body, so a recommendation to consume yeast breads in addition to vegetables can help to rectify the slight imbalances that may exist.
Most people who even have an abnormal PO4 blood test will generally be in good overall health.
If there are any personal concerns about how phosphorus levels may interact with personal health, then be sure to schedule an appointment with a medical provider right away to discuss this guide and what they mean for personal results.
Why Will The Po4 Blood Test Be Ordered
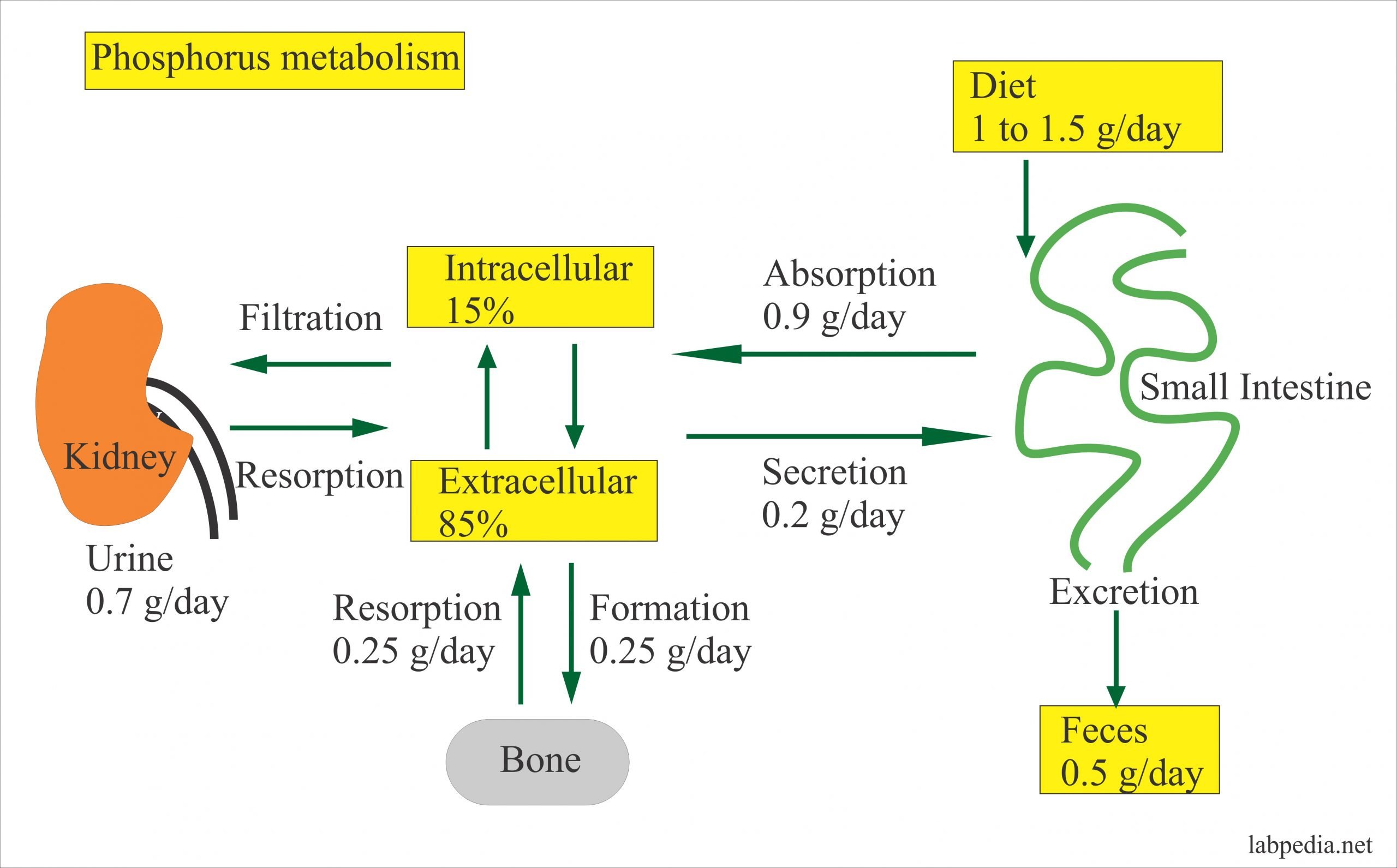
Many people can have slightly abnormal phosphorus levels consistently and never experience any physical symptoms because of it. Patients tend to arrive at the doctors office because theyve been feeling muscle weakness, cramping, or fatigue that has become bothersome. That is why the calcium blood test is typically ordered first those are primary symptoms of a calcium deficiency.
Phosphorus testing is also ordered to monitor certain kidney and digestive tract disorders. Once an abnormality in the PO4 blood test is discovered, regular testing will generally be ordered to monitor the condition and remain in place until levels stabilize. It may also be part of a diabetic treatment plan or may be ordered when an acid/base imbalance is suspected.
People who have uncontrolled diabetes that cannot be detected by other means for whatever reason may be able to have their condition diagnosed with this blood test. There may also be a timed urine collection required with the blood test to help supplement the results that are produced.
Recommended Reading: How Do Bees Fly Physics
What Does Po4 Mean In Chemistry
This page is about the meanings of the acronym/abbreviation/shorthand PO4 in the Academic & Science field in general and in the Chemistry terminology in particular.
Phosphate
Find a translation for Phosphate in other languages:
Select another language:
- Indonesia
Nearby & related abbreviations:
Discuss this PO4 abbreviation with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.
What Is Gold Mainly Used For
Today, gold still occupies an important place in our culture and society we use it to make our most prized objects: wedding rings, Olympic medals, money, jewellery, Oscars, Grammys, crucifixes, art and many more. 1. My precious: Gold has been used to make ornamental objects and fine jewellery for thousands of years.
Recommended Reading: John Thomas Child Of Rage
Lithium Iron Phosphate Battery
| around 200 W/kg |
| Energy/consumer-price |
|---|
| 3.2 V |
The lithium iron phosphate battery or LFP battery is a type of lithium-ion battery using lithium iron phosphate as the cathode material, and a graphiticcarbon electrode with a metallic backing as the anode. The energy density of LiFePO4 is lower than that of lithium cobalt oxide , and also has a lower operating voltage. The charge-discharge profiles of LFP cells are typically very flat. The main drawback of LiFePO4 is its low electrical conductivity. Therefore, all the LiFePO4 cathodes under consideration are actually LiFePO4/C . Because of low cost, low toxicity, well-defined performance, long-term stability, etc. LiFePO4 is finding a number of roles in vehicle use, utility scale stationary applications, and backup power. LFP batteries are cobalt-free.
How Do You Make Calcium Phosphate Fertilizer
Method of producing calcium phosphate fertilizer from a calcium-containing material, reactive with diluted phosphoric acid to create calcium phosphate fertilizer, calcium silicate and diluted phosphoric acid consisting of mixing the fine calcium-containing material with diluted phosphoric acid containing by weight of H3PO4 at least 30 to 40 per cent.
Don’t Miss: Prentice Hall Gold Geometry Answer Key
More Information Onmolar Mass And Molecular Weight
In chemistry, the formula weight is a quantity computed by multiplying the atomic weight of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom in the formula by the formula weight and multiplying by 100.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
Finding molar mass starts with units of grams per mole . When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
How Does Magnesium Phosphate React With Hydrochloric Acid
When reacted with hydrochloric acid, magnesium phosphate gives magnesium chloride and phosphoric acid as the products.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Recommended Reading: Who Is Generally Recognized As The Founder Of American Psychology
Occurrence Of Calcium Phosphate
Calcium and phosphorus make up the bulk of animal mineral nutrient requirements , hence DCPD is a popular and commonly used animal supplement. Dicalcium Phosphate Dihydrate is also of concern as it is the most soluble of barely soluble calcium phosphate crystals, making it a good choice for rock phosphate dissolution tests. The fate of DCPD in the soil is rather temporary.
Mineral phosphorus is usually applied to soil in a water-soluble form, such as triple super phosphate or diammonium phosphate. As phosphorus dissolves a high concentration of solution P, precipitation reactions are preferred. Calcium phosphates are found in nature in many ways and are the primary minerals for the manufacture of phosphate fertilizers and for a variety of phosphorus compounds.
Uses Of Calcium Phosphate 2
- Calcium phosphate is used as an antacid and dietary supplement in veterinary medicine.
- It is used in medicine as calcium replenisher.
- It is used as a buffer in food.
- It is used stabilizer in plastics.
- It is used in the manufacturing of milk glass.
- It is used in the production of fertilizers.
- It is used in the manufacture of luminescent materials.
- It is used to clarify sugar syrups.
- It is used in dental powders.
Don’t Miss: Kendall Hunt Geometry Answers
Uses Of Magnesium Phosphate Mg32
- Used as a source for both magnesium and phosphorus. It is used in minimal supplement.
- Used in the prevention of vitamin E deficiency.
- Used in foods at levels not to exceed current good manufacturing practice. The ingredient also may be used in infant formula.
- Used as a nutrient, pH control agent and stabilizer.
Calcium Phosphate Solubility Ca32
Solubility is one of the most important characteristics of calcium phosphate salts. It is solubility that defines the course of several reactions involving calcium phosphates such as absorption, precipitation, hydrolysis and phase transformation. Calcium phosphate solubility also plays a major role in biological processes including hard tissue formation and resorption as well as pathological calcification.
Calcium phosphate-based bone graft replacements are bioceramics that have the greatest resemblance to bone minerals. This is what makes calcium phosphate excellent biocompatibility, biodegradability and osteoconductivity.
Calcium is a mineral found naturally in food. Calcium is essential for many normal functions of the body, in particular bone formation and maintenance. Calcium can also bind to other minerals and help to extract them from the body. Calcium phosphate is used to prevent and treat calcium deficiencies. Calcium phosphate may also be used for purposes not specified in this drug guide.
Read Also: Prentice Hall Geometry Teaching Resources Answers Chapter 1
What Do The Po4 Blood Test Results Mean
What If Your Test Results Come Back High?Most people who have the PO4 blood test will have a result that comes back as normal or slightly abnormal. Test results that are higher than normal can indicate a number of different disorders. A medial provider may suspect any of the following conditions based on an individuals unique medical history.
1. Kidney disease or liver failure.2. Hyperparathyroidism.3. Ketoacidosis due to diabetic mellitus when it is first observed.
What If Your Test Results Come Back Low?It is more common to have a low test result from the PO4 blood test than a high result. Interestingly enough, health issues like diabetic ketoacidosis and hyperparathyroidism can also cause low phosphorus levels. There may be these additional health concerns evaluated with low test results as well.
1. Malnutrition, alcohol abuse, or too much calcium in the blood.2. Severe burns.3. Long-term antacid use, vitamin D deficiencies, or the overuse of diuretics.
What Are the Health Effects of Low or High Levels?High levels of phosphorus require a much faster medical response than low levels. Too much phosphorus in the body can lead to calcification, which then creates organ damage. Calcium phosphate leaves deposits in organ tissues and causes them to stop functioning properly. In rare cases, it may even lead to heart disease.