Q: K: Extent: Of: Rxn: Graphpng
As a graphical convenience , we change the x-axis to Extent of Reaction which goes from 0 for the beginning to 1 for the end . The y-axis is going to be in Free Energy, G, which is chemical potential energy. When you get to electrochemistry, you will find that it is directly related to the electromotive force, , or electrochemical potential. The free energy surface is sort of a sagged line being held at:
1) 1 on the x-axis and at G° on the free energy/y-axis, and
2) 0 on the x-axis and at G° on the free energy/y-axis.
The math describing this free energy function does not lend itself easily to a spreadsheet, except for something like an isomerization that has a small G°rxn. So, as I am using PowerPoint with its limited curved line shaping tools, I find it necessary to describe the surface as being described by a “magic rope” held by the “posts” of the Extent of Reaction, 0 and 1 respectively.
The other special point on the free energy surface is where Q = 1, that is at standard state. If the “magic rope” works correctly, the slope of the tangent at Q = 1 will be the G°rxn. Again, PowerPoint lines do not quite curve like our “magic rope”, so though I have Q = 1 at the point where the tangent on the surface is the same as G°rxn, Q = 1 is not precisely in the middle between Extent of Reaction = 0 and 1.
As a little added feature for those who find the image above a bit busy, a cleaner version is below .
The Power Of Transformer Models
In 2018, we created a state-of-the-art online platform called RXN for Chemistry using Natural Language Processing architectures in synthetic chemistry to predict the outcome of chemical reactions. Specifically, we used Molecular Transformer, where chemical reactions are represented by a domain-specific language called SMILES, or Simplified Molecular Input Line Entry System, is a notation system for representing molecules and reactions.
Guide Students As You Answer The Following Question Together:
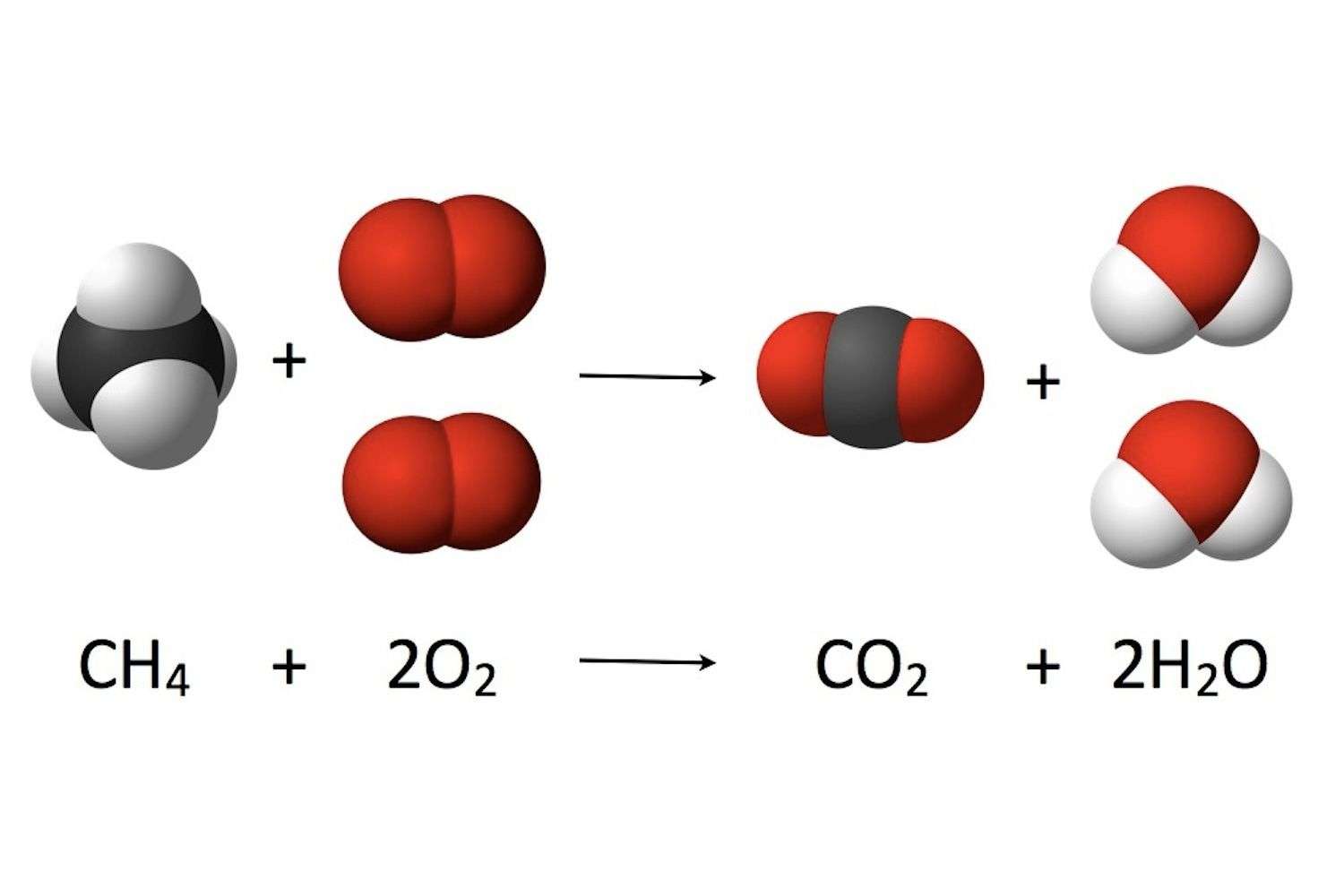
- How many carbon, hydrogen, and oxygen atoms are in the reactants compared to the number of carbon, hydrogen, and oxygen atoms in the products?
- Show students how to use the big number in front of the molecule and the little number after an atom of the molecule to count the atoms on both sides of the equation. Explain to students that the subscript tells how many of a certain type of atom are in a molecule. The coefficient tells how many of a particular type of molecule there are. So if there is a coefficient in front of the molecule and a subscript after an atom, you need to multiply the coefficient times the subscript to get the number of atoms.
- For example, in the products of the chemical reaction there are 2H2O. The coefficient means that there are two molecules of water. The subscript means that each water molecule has two hydrogen atoms. Since each water molecule has two hydrogen atoms and there are two water molecules, there must be 4 hydrogen atoms.
| Atoms |
|---|
| Hydrogen |
| Oxygen |
Also Check: Hrw Algebra 1
Ibm Rxn For Chemistry: Unveiling The Grammar Of The Organic Chemistry Language
In Extraction of organic chemistry grammar from unsupervised learning of chemical reactions, RXNMapper extracts the grammar of organic chemistry.
In Extraction of organic chemistry grammar from unsupervised learning of chemical reactions, RXNMapper extracts the grammar of organic chemistry.
Talk to any organic chemist, and they will tell you that learning organic chemistry is like learning a new language, with grammar similar to a myriad of chemical reaction rules. And its is also about intuition and perception, much like acquiring a language as a child.
In our paper Extraction of organic chemistry grammar from unsupervised learning of chemical reactions , published in the peer-reviewed journal Science Advances, scientists from IBM Research Europe, the MIT-IBM Watson AI Lab, and the University of Bern for the first time extracted the grammar of organic chemistrys language from a large number of organic chemistry reactions.1 For that, we used RXNMapper-Vis, a cutting-edge, open-source atom-mapping tool we developed. RXNMapper performs better than the current commercially available tools, and learns without human supervision.2
Review What Happens During A Physical Change And Introduce The Idea Of Chemical Change

Tell students that in previous chapters they have studied different aspects of physical change. When atoms and molecules speed up or slow down, that is a physical change. When they change state from liquid to solid or from gas to liquid, that is a physical change. When a substance is dissolved by water or some other solvent, a new substance has not really been formed. The ions or molecules can still come back together to form the original substance.
Let students know that in this chapter they will explore what happens during a chemical change. In a chemical change, the atoms in the reactants rearrange themselves and bond together differently to form one or more new products with different characteristics than the reactants. When a new substance is formed, the change is called a chemical change.
Don’t Miss: Klohe Kardashians Real Father
Reactions That Produce Gases Such As Oxygen Or Carbon Dioxide
Hydrogen peroxide decomposes to produce oxygen:
2\text_2\text_2\rightarrow 2\text_2\text+\text_2
The volume of oxygen produced can be measured using the gas syringe method. The gas collects in the syringe, pushing out against the plunger. The volume of gas that has been produced can be read from the markings on the syringe. This change in volume can be converted to a change in concentration , and dividing this by the time of the reaction will yield an average reaction rate.
Gas syringe method: In a reaction that produces a gas, the volume of the gas produced can be measured using the gas syringe method.
What Is Mol Rxn Units In Thermochemistry Calculations
I pulled the following chemical equation from the CollegeBoard AP® Chemistry Course and Exam Description, Fall 2020:
|
2SO2 + O2 2SO3 H° = -198 kJ/mol-rxn |
This equation means that:
when two moles of gaseous sulfur dioxide react with one mole of gaseous oxygen in standard conditions of 298 K and atmospheric pressure, the reaction yields two moles of gaseous sulfur trioxide and releases 198 kJ of excess energy.
While the chemical equation itself is standard, the notation for the reactions change in enthalpy is nuanced and can be a sore point sowing confusion. Lets break the enthalpy term down piece by piece:
H denotes a state of enthalpy.
H denotes a change in enthalpy and implies that:
Because H is negative in this reaction, the products have a lower enthalpy than the reactants this reaction releases energy and the released energy would be observed as excess heat emerging from the source. All to say that the reaction is exothermic.
The prime or degree mark in H° denotes standard reaction conditions, such that the reaction is conducted at 298 K and 1 atmosphere of pressure.
H° = -198 kJ denotes that the reaction, with the written stoichiometry and conducted at standard reaction conditions, releases exactly 198 kJ of energy.
Don’t Miss: Geometry Segment Addition Postulate Worksheet Answers
The Context Of Chemical Reactions
Chemical reactions happen all around us every day. Whether it is a single replacement reaction in the battery of our flashlight, a synthesis reaction that occurs when iron rusts in the presence of water and oxygen, or an acid-base reaction that happens when we eat we experience chemical reactions in almost everything we do. Understanding these reactions is not an abstract concept for a chemist in a far off laboratory, rather it is critical to understanding life and the world around us. To truly master chemical reactions, we need to understand the quantitative aspect of these reactions, something referred to as stoichiometry, and a concept we will discuss in another module.
Dalton: Law Of Multiple Proportions
The English chemist John Dalton helped make sense of the laws of conservation of mass and definite proportions in 1803 by proposing that matter was made of atoms of unique substances that could not be created or destroyed .
Dalton extended Prousts ideas by recognizing that it was possible for two elements to form more than one compound, but that whatever the compound was, it would always contain elements combined in whole number ratios . This observation is known as the law of multiple proportions and with his atomic theory, helped to cement Lavoisiers observations.
Figure 5
These advancements, taken together, laid the groundwork for our modern understanding of chemical reactions, chemical equations, and chemical stoichiometry, or the process of expressing the relative quantities of reactants and products in a chemical reaction.
Comprehension Checkpoint
____ first theorized that while substances changed form during a chemical reaction, the mass of the system did not change.
Recommended Reading: Holt Geometry Homework And Practice Workbook Answer Key
What Happens To Chemical Bonds When A Chemical Reaction Takes Place
According to the modern view of chemical reactions, bonds between atoms in the reactants must be broken, and the atoms or pieces of molecules are reassembled into products by forming new bonds. Energy is absorbed to break bonds, and energy is evolved as bonds are made. In some reactions the energy required to break bonds is larger than the energy evolved in making new bonds, and the net result is the absorption of energy. Hence, different types of bonds may be formed in a reaction. A Lewis acid-base reaction, for example, involves the formation of a covalent bond between a Lewis base, a species that supplies an electron pair, and a Lewis acid, a species that can accept an electron pair. Ammonia is an example of a Lewis base. A pair of electrons located on a nitrogen atom may be used to form a chemical bond to a Lewis acid.
Reactions At The Solid
Reaction can take place at the solid|gas interface, surfaces at very low pressure such as ultra-high vacuum. Via scanning tunneling microscopy, it is possible to observe reactions at the solid|gas interface in real space, if the time scale of the reaction is in the correct range. Reactions at the solid|gas interface are in some cases related to catalysis.
Recommended Reading: Molecular Geometry Of Ccl4
What Causes A Chemical Reaction To Begin
The atoms and molecules generated by a chemical reaction are referred to as products. There are no new atoms produced, and there are no atoms destroyed. Reactants come into contact with each other in a chemical reaction, links between atoms in the reactants are broken, and atoms rearrange and establish new bonds to produce the products.
How Are Chemical Reactions Classified
Chemists classify chemical reactions in a number of ways: by type of product, by types of reactants, by reaction outcome, and by reaction mechanism. Often a given reaction can be placed in two or even three categories, including gas-forming and precipitation reactions. Many reactions produce a gas such as carbon dioxide, hydrogen sulfide, ammonia, or sulfur dioxide. Cake batter rising is caused by a gas-forming reaction between an acid and baking soda . Classification by types of reactants include acid-base reactions and oxidation-reduction reactions, which involve the transfer of one or more electrons from a reducing agent to an oxidizing agent. Examples of classification by reaction outcome include decomposition, polymerization, substitution, and elimination and addition reactions. Chain reactions and are examples of classification by reaction mechanism, which provides details on how atoms are shuffled and reassembled in the formation of products.
chemical reaction, a process in which one or more substances, the reactants, are converted to one or more different substances, the products. Substances are either chemical elements or compounds. A chemical reaction rearranges the constituentatoms of the reactants to create different substances as products.
Recommended Reading: Who Is Paris Jackson’s Mother
Lavoisier: Law Of Mass Conservation
Antoine Lavoisier was a French nobleman in the 1700s who began to experiment with different chemical reactions. At the time, chemistry still couldnt be described as being a true, quantitative science. Most of the theories that existed to explain the way that substances changed relied upon Greek philosophy, and there was precious little experimental detail attached to the alchemists tinkering.
However, during the second half of the 18th century, Lavoisier performed many quantitative experiments and observed that while substances changed form during a chemical reaction, the mass of the system or a measure of the total amount of stuff present did not change. In doing so, Lavoisier championed the idea of conservation of mass during transformations . In other words, unlike the alchemists before him who thought that they were creating matter out of nothing, Lavoisier proposed that substances are neither created nor destroyed, but rather change form during reactions. Lavoisiers ideas were published in the seminal work Traité élémentaire de Chimie in 1789 , which is widely hailed as the birth of modern chemistry as a quantitative science.
Figure 3
Reaction Kinetics Of Canola Oil Methyl Ester Via Ultrasonic
Chemical kinetics is the study of the rates of chemical reactions, factors which are influential in the rates and the explanation of the rates with respect to the reaction mechanisms of chemical processes . Chemical kinetics and reactor design are of primary concern in the exploitation of chemical reactions in industrial production . From an economic point of view, it is also a crucial factor in the success or failure of a chemical plant. Unlike in thermodynamics, where the concern is expressed in the change of reactions without considering the intermediate states or time, in chemical kinetics, the rate of change of the concentration of reactants or products is followed by time. Therefore the rate of chemical reactions, as well as the effect of various factors such as concentration, temperature, catalyst, and so forth, are determined quantitatively . Fig. 3.6 shows the reaction kinetics of the pseudo first order for canola methyl ester synthesis under ultrasonic-assisted conditions. The kinetics has been performed at three different temperatures. The reaction rate constants were found to be 0.0666 min1 at 45°C, 0.0929 min1 at 50°C, and 0.1044 min1 at 55°C. Fig. 3.7 reveals the activation energy of 39.05 kJ/mol and frequency factor of 12×107 min1.
Figure 3.6. Pseudo first-order reaction kinetics fitting for canola-based methyl ester.
Figure 3.7. Activation energy and frequency identification plot.
D.J. Donaldson, in, 2003
Don’t Miss: Elastic Force Definition Physics
What Is A Chemical Reaction
A chemical reaction is in which the bonds are broken within reactant molecules, and new bonds are formed within product molecules in order to form a new substance.
Chemical reactions are all around us, from the metabolism of food in our body to how the light we get from the sun is the results of chemical reactions. Before beginning with chemical reactions, it is important to know about physical and chemical changes.
A burning candle is the best example of physical and chemical change. Take a candle and light it. As time passes, we can observe that the candle changes to wax. If you cover the candle with a jar, it will extinguish.
In the demonstration, burning of the candle is a chemical change while conversion of the candle to wax is a physical change. In a physical change, there is basically a change of state of the substance but in the case of a chemical change mostly a new substance is formed in which either energy is given off or absorbed. Thus, we can conclude that chemical changes are accompanied by certain physical changes.
What Does Rxn Stand For
What does RXN mean? This page is about the various possible meanings of the acronym, abbreviation, shorthand or slang term: RXN.
Filter by:
Popularity rank for the RXN initials by frequency of use:
Couldn’t find the full form or full meaning of RXN?
Maybe you were looking for one of these abbreviations:
Discuss these RXN abbreviations with the community:
Report Comment
We’re doing our best to make sure our content is useful, accurate and safe.If by any chance you spot an inappropriate comment while navigating through our website please use this form to let us know, and we’ll take care of it shortly.
Read Also: Chapter 7 Test Form A Answer Key
What Is Delta H Rxn In Chemistry
4.8/5rxnreaction
Correspondingly, what is Delta H chemistry?
In chemistry, the letter “H” represents the enthalpy of a system. Enthalpy refers to the sum of the internal energy of a system plus the product of the system’s pressure and volume. The delta symbol is used to represent change. Therefore, delta H represents the change in enthalpy of a system in a reaction.
Also, what is Delta E and Delta H? The question is “Under what circumstances are delta E& delta H essentially equal. Using the equation delta H = delta E + PV. Delta E& H will essentially be equal when there is no change in volume.
Also, how do you find Delta H RXN?
Use the formulaH = m x s x T to solve.Once you have m, the mass of your reactants, s, the specific heat of your product, and T, the temperature change from your reaction, you are prepared to find the enthalpy of reaction. Simply plug your values into the formulaH = m x s x T and multiply to solve.
What does Q stand for in chemistry?
The reaction quotient Q is a measure of the relative amounts of products and reactants present in a reaction at a given time.