What Is Diamagnetism In Simple Terms
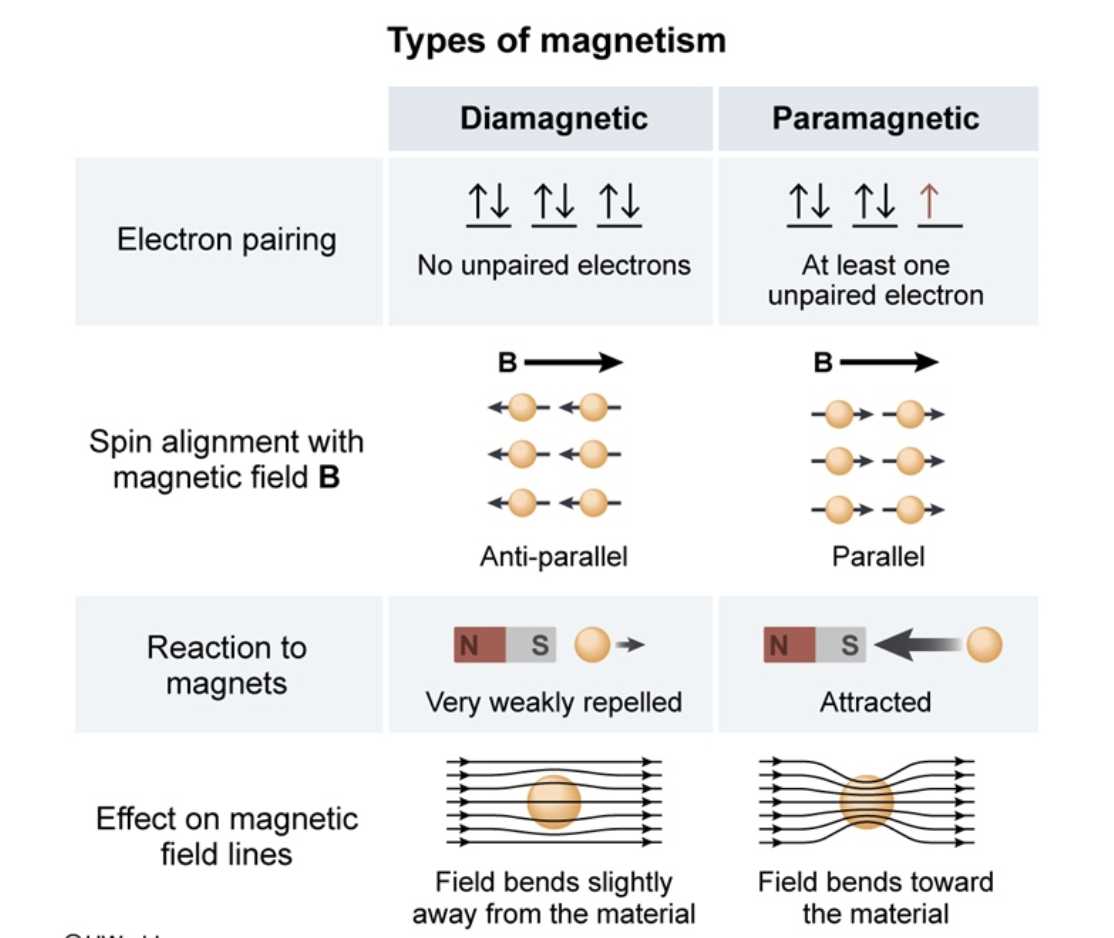
Diamagnetism is the property of an object or material that causes it to create a magnetic field in opposition to an externally applied magnetic field. It is a quantum mechanical effect that occurs in all materials where it is the only contribution to the magnetism the material is called a diamagnet.
Walter Kohn: Nobel Laureate
Walter Kohn is a theoretical physicist who studies the electronic structure of solids. His work combines the principles of quantum mechanics with advanced mathematical techniques. This technique, called density functional theory, makes it possible to compute properties of molecular orbitals, including their shape and energies. Kohn and mathematician John Pople were awarded the Nobel Prize in Chemistry in 1998 for their contributions to our understanding of electronic structure. Kohn also made significant contributions to the physics of semiconductors.
Figure 6. Walter Kohn developed methods to describe molecular orbitals.
Kohns biography has been remarkable outside the realm of physical chemistry as well. He was born in Austria, and during World War II he was part of the Kindertransport program that rescued 10,000 children from the Nazi regime. His summer jobs included discovering gold deposits in Canada and helping Polaroid explain how its instant film worked. Although he is now an emeritus professor, he is still actively working on projects involving global warming and renewable energy.
What Are Paramagnetic Materials
Paramagnetic materials have some unpaired electrons due to these unpaired electrons the net magnetic moment of all electrons in an atom is not added up to zero. Hence atomic dipole exists in this case. On applying external magnetic field the atomic dipole aligns in the direction of the applied external magnetic field. In this way, paramagnetic materials are feebly magnetized in the direction of the magnetizing field.
Also Read: Ferromagnetic Materials
In simple words, we can say that these materials usually experience a weak attraction to magnets. This type of magnetism is known as paramagnetism. It occurs mainly due to the presence of unpaired electrons in the material or due to the partial alignment of randomly oriented atomic dipole along the field.
Paramagnetism can further be of two types.
- In the first type, the magnetic moments are found in low concentrations which leads to its separation from one another. Their spins also do not interact.
- In the second type, paramagnetism occurs due to the interactions between the magnetic moment. In this case. the interactions are very weak. Therefore, there is no net magnetization when the applied field is zero.
This type of magnetization is based on Curies law. According to the law, paramagnetic materials magnetic susceptibility is inversely proportional to their temperature. It is represented as
M = H = C/T x H
Where,
H = auxiliary magnetic field.
Read Also: Chapter 3 Test Algebra 2 Answers
Important Points To Remember
- The magnetization of paramagnetic substances is inversely proportional to absolute temperature. It means that if we increase the temperature, paramagnetic substances start losing their magnetic power.
- On lowering the temperature a stage comes when 100 atomic dipoles align with the external magnetic field this state is called a saturation state after this curies law is invalid.
- Paramagnetism is executed by solids liquids and gases.
Also Read: Diamagnetic Materials
Computational Chemistry In Drug Design
While the descriptions of bonding described in this chapter involve many theoretical concepts, they also have many practical, real-world applications. For example, drug design is an important field that uses our understanding of chemical bonding to develop pharmaceuticals. This interdisciplinary area of study uses biology to identify specific targets, such as a binding site that is involved in a disease pathway. By modeling the structures of the binding site and potential drugs, computational chemists can predict which structures can fit together and how effectively they will bind . Thousands of potential candidates can be narrowed down to a few of the most promising candidates. These candidate molecules are then carefully tested to determine side effects, how effectively they can be transported through the body, and other factors. Dozens of important new pharmaceuticals have been discovered with the aid of computational chemistry, and new research projects are underway.
Figure 7. The molecule shown, HIV-1 protease, is an important target for pharmaceutical research. By designing molecules that bind to this protein, scientists are able to drastically inhibit the progress of the disease.
You May Like: Fsa Algebra 1 Eoc Answer Key
Paramagnetism: Definition Properties And Examples
Paramagnetic materials are attracted towards magnetic fields in which they move from a region of a weaker magnetic field to a stronger magnetic field this phenomenon is called paramagnetism. These paramagnetic materials consist of most of the elements and some compounds that have unpaired electrons in their valence shells. In this article, we will learn about paramagnetism by learning about paramagnetic materials, their examples, and the process of paramagnetism.
|
Table of Content |
Do Recycling Companies Make Money
The cost of processing any commodity at a recycling facility is about $75 per ton. In the case of cardboard, each ton would earn a profit of $50 per ton, to be split by the municipality and recycling company. Paper would earn a profit of $5 per ton, to be split by the municipality and recycling company.
Recommended Reading: Molecular Geometry Ccl4
The Magnetization Of Solids
If a solid substance is placed in an applied magnetic field, you might expect the behavior of the molecules in the substance to depend to some extent on the state of the material. That is, a gas, which has molecules that move about quite freely, and a liquid, in which molecules remain together but are free to slide past each other, might behave differently than a solid, whose molecules are locked in place, usually in a lattice-type structure.
If you picture a solid’s basic crystal structure , you can imagine the nuclei of the atoms being at the centers of cubes, with the electrons occupying spaces in between, free to vibrate and, in the case of metal solids, free to roam about unchained to their parent nuclei.
When the electrons of a solid render the substance a permanent magnet or one that can be made into such a magnet, the substance is called ferromagnetic . In addition to iron, the elements cobalt, nickel and gadolinium are ferromagnetic.
Most substances, however, exhibit other responses to magnetic fields, making most atoms paramagnetic or diamagnetic. These properties can be found to different degrees in the same materials, and factors such as temperature can affect a material’s response to applied magnetic fields.
Characteristics Of Paramagnetic Compounds And Atoms
Paramagnetic elements and paramagnetic molecules share one main trait and that is having unpaired electrons. The more of these there are, the more likely the atom or molecule is to show paramagnetism. This is because these electrons align themselves in a fixed way with the orientation of an applied magnetic field, creating something called magnetic dipole moments around each atom or molecule.
If you are familiar with electron “filling” rules, you know that orbitals within subshells can hold two electrons each, and that there is one of these for an s subshell, three for a p subshell and five for a d subshell. This allows for a capacity of two, six and 10 electrons in each subshell, but these will fill up so that each orbital holds just one electron for as long as possible until the one electron there has to accommodate a neighbor.
This means that you can use the information in a periodic table of the elements to determine if a material will be paramagnetic, and happily, whether it will be weakly paramagnetic or strongly paramagnetic .
Also Check: Eoc Fsa Warm Ups Algebra 1 Answers
What Is The Meaning Of The Term Paramagnetic
Paramagnetic materials have a small, positive susceptibility to magnetic fields, and are very weakly attracted by an externally applied magnetic field.
These materials do not retain the magnetic properties when the external field is removed. Paramagnetic materials include magnesium, molybdenum, lithium, and tantalum.
Paramagnetic properties are due to the presence of some unpaired electrons, and from the realignment of the electron paths caused by the external magnetic field.
Small stainless steel particles can become paramagnetic, which means that they can be picked up by strong magnets.
Image Source: Boundless. a href=”https://www.boundless.com/physics/textbooks/boundless-physics-textbook/magnetism-21/applications-of-magnetism-160/paramagnetism-and-diamagnetism-566-6306/”> Paramagnetism and Diamagnetism.Boundless Physics. Boundless, 21 Jul. 2015. Retrieved 08 Sep. 2015.
How Do We Decide If A Substance Is Paramagnetic Or Diamagnetic
Electron spin generates a spin magnetic moment that determines whether a substance is diamagnetic or paramagnetic.
Explanation:
An electron behaves as if it were spinning on its axis. The electron bears a charge, and a charge in motion is an electric current.
We learn in physics that an electric current generates a magnetic field. So an electron behaves like a tiny magnet.
An electron has a spin magnetic moment that can line up with or against an applied magnetic field. Lining up with the field is the lower energy state. It corresponds to #m_s#
If all electrons in an atom are paired, then the number of electrons with #m_s# = +½ is the same as the numbers with #m_s# = -½. The magnetic moments cancel, and the substance is diamagnetic.
Thus, NaCl is diamagnetic, because all spins are paired in Na and in Cl.
If an atom has one or more unpaired electrons, the magnetic dipoles of the unpaired electrons will line up with an applied magnetic field. The substance will be paramagnetic.
For example, the electron configuration of nickel is # 4s^2 3d^8# . Nickel has two unpaired electrons, so it is paramagnetic.
Note: an atom has other magnetic moments besides those caused by electron spin.
You May Like: Who Are Paris Jackson’s Biological Parents
Hybridization Mot And Paramagnetism
In what way can hybridization or molecular orbital theory be used to explain paramagnetism?
For instance, when something is hybridized to make enough bonding electrons, do all the electrons end up paired? Or are some left unpaired, explaining paramagnetism?
Paramagnetism is a form of magnetism whereby certain materials are attracted by an externally applied magnetic field. Valence bond theory and hybridisation doesn’t really do a good job at predicting whether a molecule is paramagnetic or diamagnetic . This is why molecular orbital theory is so useful as it is successful at predicting whether a molecule is paramagnetic.
For a molecule to be paramagnetic, it needs to have an overall magnetic moment meaning that it needs an unpaired electron. If all the electrons are paired, then the molecule is diamagnetic. So by seeing whether a molecule has an unpaired electron, we can predict if it is paramagnetic or not.
Now lets consider the example of $\ce$. Experimentally, $\ce$ is known to paramagnetic. According to VBT, $\ce$ should look like this:
As you can see, all the electrons are paired. Therefore VBT predicts that $\ce$ should be diamagnetic.
Now that we got the MOs, all we have to do is fill them with electrons using the same method that we use for AOs. By doing that we get:
Note that we have 2 unpaired electrons. Therefore MOT correctly predicts that $\ce$ should be paramagnetic, unlike VBT which predicts that $\ce$ is diamagnetic.
Chemistry End Of Chapter Exercises
molecular orbitals and molecular orbitals
for an atomic orbital and for a molecular orbital
bonding orbitals and antibonding orbitals
Na22+
- molecular orbital in which the electron density is found along the axis of the bond
- * bonding orbital
- antibonding molecular orbital formed by out-of-phase overlap of atomic orbital along the axis of the bond, generating a node between the nuclei
You May Like: Algebra Chapter 3 Test Answers
Can Stainless Steel Be Recycled
Steel is the most recycled material on the planet. When stainless steel is recycled and melted down, these valuable alloys are able to be extracted and reused with no degradation in performance from product to product. In fact, the vast majority of stainless steel is manufactured using previously recycled materials.
How To Tell If An Element Is Paramagnetic Or Diamagnetic
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Materials may be classified as ferromagnetic, paramagnetic, or diamagnetic based on their response to an external magnetic field.
Ferromagnetism is a large effect, often greater than that of the applied magnetic field, that persists even in the absence of an applied magnetic field. Diamagnetism is a property that opposes an applied magnetic field, but it’s very weak.
Paramagnetism is stronger than diamagnetism but weaker than ferromagnetism. Unlike ferromagnetism, paramagnetism does not persist once the external magnetic field is removed because thermal motion randomizes the electron spin orientations.
The strength of paramagnetism is proportional to the strength of the applied magnetic field. Paramagnetism occurs because electron orbits form current loops that produce a magnetic field and contribute a magnetic moment. In paramagnetic materials, the magnetic moments of the electrons don’t completely cancel each other out.
Read Also: Who Are Paris Jackson’s Biological Parents
Systems With Minimal Interactions
The narrowest definition would be: a system with unpaired spins that do not interact with each other. In this narrowest sense, the only pure paramagnet is a dilute gas of monatomic hydrogen atoms. Each atom has one non-interacting unpaired electron.
A gas of lithium atoms already possess two paired core electrons that produce a diamagnetic response of opposite sign. Strictly speaking Li is a mixed system therefore, although admittedly the diamagnetic component is weak and often neglected. In the case of heavier elements the diamagnetic contribution becomes more important and in the case of metallic gold it dominates the properties. The element hydrogen is virtually never called ‘paramagnetic’ because the monatomic gas is stable only at extremely high temperature H atoms combine to form molecular H2 and in so doing, the magnetic moments are lost , because of the spins pair. Hydrogen is therefore diamagnetic and the same holds true for many other elements. Although the electronic configuration of the individual atoms of most elements contain unpaired spins, they are not necessarily paramagnetic, because at ambient temperature quenching is very much the rule rather than the exception. The quenching tendency is weakest for f-electrons because f orbitals are radially contracted and they overlap only weakly with orbitals on adjacent atoms. Consequently, the lanthanide elements with incompletely filled 4f-orbitals are paramagnetic or magnetically ordered.
Bonding In Diatomic Molecules
A dihydrogen molecule forms from two hydrogen atoms. When the atomic orbitals of the two atoms combine, the electrons occupy the molecular orbital of lowest energy, the 1s bonding orbital. A dihydrogen molecule, H2, readily forms because the energy of a H2 molecule is lower than that of two H atoms. The 1s orbital that contains both electrons is lower in energy than either of the two 1s atomic orbitals.
A molecular orbital can hold two electrons, so both electrons in the H2 molecule are in the 1s bonding orbital the electron configuration is . We represent this configuration by a molecular orbital energy diagram in which a single upward arrow indicates one electron in an orbital, and two arrows indicate two electrons of opposite spin.
Figure 9.
A dihydrogen molecule contains two bonding electrons and no antibonding electrons so we have
Because the bond order for the HH bond is equal to 1, the bond is a single bond.
A bond order of zero indicates that no bond is formed between two atoms.
Figure 10.
Don’t Miss: What Is Figure Ground Perception Psychology
What Atom Is Always Paramagnetic And Why
Regardless of its electron configuration, it must always be paramagnetic when it’s a single, neutrally charged atom: Carbon Nitrogen Oxygen Neon Argon
At first, I eliminated D and E. Then, I tried to find the answer by checking the electron confiurations of the atoms I saw the phrase “regardless of its electron configuration”, but I didn’t know what else I can do. Then, I found out that all three atoms have some unpaired electrons
I checked charts with paramagnetic elements filled in three first pictures on google said different things , but after all the info I have found on the net I understood that C is the most likely answer. I don’t understand why I checked a few videos that said that if an atom has 1 or more unpaired electrons it is paramagnetic then, A, B, and C are all paramagnetic. A few sites said we need to look not at the elements, but at the compounds the task said, anyway, we are looking at the single, neutrally charged atom.
Please tell if it is oxygen and if yes why it is paramagnetic but other elements are not.
- 7$\begingroup$Answer is nitrogen, because it has an odd number of electrons, and therefore there must be at least one unpaired electron. For carbon and oxygen which have an even number of electrons, it’s always possible to pair them up somehow . I don’t like the question’s phrasing, but that’s what it’s going for.$\endgroup$