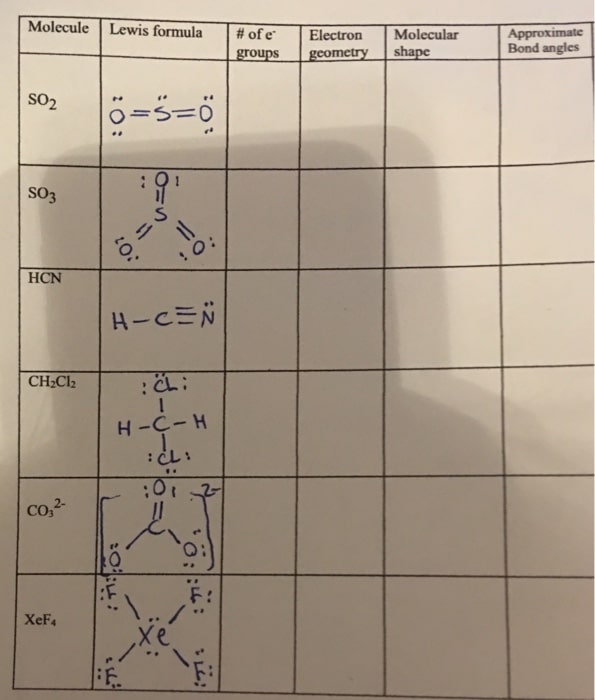
Stage : Balancing The Charges On The Compound
So a great deal of solitary sets will just make the compound unsound in nature. Subsequently, there will be extra bond matching among carbon and nitrogen.
So as carbon has two electrons left along these lines it can make 2 additional bonds with nitrogen, leaving nitrogen with just one sets of solitary electrons.
This is the most steady Lewis structure that can be made for HCN.
We trust that you found out about how the connections between HCN are made. Presently we should move to see the hybridization of the compound.
This is on the grounds that An alludes to the focal atom and X is the other adjoining atoms which is 2 on account of HCN, providing us with the recipe of AX2.
Why Hydrogen Cyanide Is Polar
In Hydrogen cyanide carbon has an electronegativity of 2.5, hydrogens electronegativity is 2.1, and
Nitrogen has an electronegativity of 3. Any molecule that has a distinction of electronegativities of any dipole second is thought of as polar.
In this way, Hydrogen cyanide is a polar molecule, comprised of three unique atoms of hydrogen, carbon, and nitrogen. It is a polar molecule with bond points of 180 degrees.
What Does Polarity Mean
Polarity is an important property of any chemical molecule. It depends on the separation of charges. This characteristic is defined by another concept called electronegativity.
For that, we can consult the Pauling Electronegativity chart:
Here, in this Pauling Electronegativity scale, we can get an idea of the trends in electronegativity value along groups and periods in the periodic table.
Electronegativity: It is an atomic property that describes its tendency to attain or gain more electrons for bond formation.
When two atoms of the same element come together to form a homogeneous diatomic molecule, due to linear arrangement and same electronegativity values , we have a non-polar molecule. Here, the net dipole is zero.
However, when two or more atoms of different elements form a molecule or there is an asymmetry in the molecular shape, we find a polar molecule where the net dipole is not equal to 0 .
Don’t Miss: What Does N Stand For In Chemistry
The Molecular Geometry Of Hydrogen Cyanide
The molecular geometry of any particular molecule contributes to the study of its three-dimensional structure, atom arrangement, and form.
Hydrogen cyanide is an AX2 molecule, where A is the central atom and X is a bonded atom with the core atom. Furthermore, according to VSEPR theory, any molecule with an AX2structure would have linear geometry.
Hence, hydrogen cyanide possesses linear molecular geometry.
Hcn Lewis Structure Molecular Geometry Hybridization And Polarity
Prussic acid is another name for hydrogen cyanide, or HCN. Its a liquid that has no color, is exceedingly combustible, and is poisonous. HCN can be found as a liquid or a gas. The components of this weak acid are carbon, hydrogen, and nitrogen. It has a linear geometry with a triple bond between carbon and nitrogen .
This blog article will help you understand the Lewis structure, hybridization, molecular geometry, and molecular orbital diagram of HCN.
Before going towards the Lewis structure, let us understand some general terms used in it.
Valence Electron
Valence electrons are the total number of electrons present in the atoms outermost shell. Only valence shell electrons involve in bond formation. These valence electrons either share or are transferred for bond formation.
Valance electrons are electrons in the outermost shell that are vital for bonding and forming molecules.
The Octet Rule states that every atom likes to have eight valence electrons because this is the most stable and low-energy state.
Read Also: How Does Geography Affect Government
Hybridization Of Carbon In Hydrogen Cyanide
Carbon is triple-bonded to nitrogen, and so there are one sigma and two pi bonds.As per the rule, the first bond between any two atoms is a sigma bond, and the second and third bonds are pi bonds)This means two p orbitals are required to be left over after hybridization.2 pi bonds = 2 leftover p orbitals.As a result, one of carbons p orbitals is available to hybridize.
Employments Of Hydrogen Cyanide
Hydrogen cyanide is utilized in the arrangement of acrylonitrile, which is utilized in the creation of acrylic strands, engineered elastic, and plastics.
Hydrogen cyanide and its compounds are utilized for some, substance processes, including fumigation, the case solidifying of iron and steel, electroplating, and the centralization of metals.
Hydrogen cyanide is a great dissolvable for some salts, however it isnt generally utilized as a dissolvable as a result of its harmfulness.
You May Like: What Are Reflexes In Psychology
S To Remember While Drawing The Lewis Structure
To draw the Lewis structure of a molecule, there are a few general steps to follow. The number of steps can be adjusted depending on the molecule or ions complexity. Well look at each stage of drawing the Lewis structure of hydrogen cyanide.
- Calculate the total number of electrons in the valence shells.
- Choosing the centre atom
- Draw lone pairs.
What Is A Mo Diagram
MO chart is only a depiction of how the substance bonds are shaped in any compound. The chart is a portrayal of various energy levels and why a compound exists in nature or why a few compounds dont exist by any stretch of the imagination.
With the assistance of this hypothesis, we can dive deeper into the interior structures, bond sharing, and different energy of orbitals of a compound.
On account of HCN, let us take a gander at how the atomic orbitals breaker to make molecular orbitals.
Electronic design of C is 2s2 2p2, electronic setup of H is 1s1, and electronic arrangement of N is 2s2 2p3. Here, one sp orbital of C wires with 1s orbital of H.
Whats more the other sp orbital of C circuits with one of the p orbitals of Nitrogen. The px orbitals of both C and N structure sigma bonds while the Py and Pz orbitals structure opposite Pi bonds.
Polarity Of HCN
Presently let us take a gander at whether the compound is polar or non polar in nature. Allow us first to discover what is the electronegativity of every atom here.
Carbon has an electronegativity of 2.55, for hydrogen, the electronegativity is 2.2 and for nitrogen, the electronegativity is 3.04.
As you can see that there is anything but a tremendous contrast between the electronegativity of carbon and nitrogen yet this little distinction gives solid outcomes.
Recommended Reading: How To Calculate Precision Physics
Why Is The Shape Of Hcn Linear While That Of H2o Angular
Hydrogen cyanide is a linear molecule as all the bonded atoms lie symmetrically, in the same plane and there is no lone pair on the central C atom.
Contrarily, water is an angular or bent-shaped molecule because of 2 lone pairs situated on the central O atom in the molecule.
Lone pair-lone pair repulsions and lone pair-bond pair repulsions disturb the symmetry of the three bonded atoms. The bond angle decreases, and the molecule adopts an asymmetric angular shape.
Also Read:-
Stage : Now We Will Draw The Lewis Spot Structure Of The Compound
Presently you can see that the focal atom here is Carbon since it is simple for Carbon to become steady as it is the most un-electronegative of all.
Notwithstanding, hydrogen is the most un-electronegative however it cannot be a focal atom since it has just one extra electron.
The other two atoms H and N are joined to C by a single bond. To make the portrayal clean we need to show the excess solitary pair of electrons on the atoms too after the underlying bonds are made.
Here, after two electrons carbon share with hydrogen and nitrogen every, it is left with 2 additional electrons in the external shell.
The of hydrogen is finished so there are no solitary sets on it.
Whats more for nitrogen, subsequent to offering one electron to carbon it is left with 4 electrons which implies there are 2 solitary sets of electrons on it.
Also Check: How Does Psychology Explain Human Behavior
How Many Lone Pairs Are In A Lewis Structure Of Hcn
Lewis structure of hydrogen cyanide contains a single covalent bond between carbon and hydrogen and a triple bond between nitrogen and a carbon atom. It has ten electrons, out of which 8 participate in bonding while two are present as lone pairs on the terminal nitrogen atom. Therefore, the HCN structure possesses one lone pair on the nitrogen atom.
How To Draw The Lewis Structure Of Oxygen

In theO2 Lewis structure, there is a double bond between two oxygen atoms.Oxygen is a diatomic nonpolar molecule with a bond angle of 180 degrees.In its molecule, both oxygen atoms have the same electronegativity value, both atoms share equal ratios of bonded shared electrons, and the overall O2 molecule turns out to be nonpolar.
Don’t Miss: What Is The Molecular Geometry Of Hcn
What Are The Bond Angles And Polarity Of Hcn
Once we know the Lewis structure and Molecular Geometry of any molecule, it is easy to determine its bond angles and polarity. As this molecule has a linear molecular geometry, HCN has bond angles of 180 degrees.
What is the molecular geometry of a triiodide ion?
Although the molecular geometry is linear as discussed earlier, the electronic geometry is trigonal bipyramidal. The structure of triiodide ions is such that the middle atom carries the – charge. Now, as we discuss polarity, let us define it. In chemistry, polarity means something that will bear a non-zero value of dipole moment.
Previous Post
Questions About Hcn Molecular Geometry
If you have any questions about Hcn Molecular Geometry Please let us know, all your questions or suggestions will help us improve in the following articles!
The article HCN Molecular Geometry & Bond Angles was compiled by me and my team from many sources. If you find the article Hcn Molecular Geometry helpful to you, please support the team Like or Share!
Read Also: What Is Fixed Ratio In Psychology
Uses Of Hydrogen Cyanide
- HCN is an important industrial chemical, with over a million tons of production per year worldwide.
- Hydrogen cyanide serves as a fumigant to prevent pests.
- Its used in the manufacturing of plastics, paints, synthetic fibres, and many other chemicals.
- HCN also has uses in metal cleaning and ore extraction.
- used in electroplating, printing, dying, and photography.
Molecular Geometry For Hcn
- Molecular geometry for HCN- Drawing of Lewis structure
Draw the Lewis structure & Molecular geometry for HCN: explain the configuration in the molecule of valence electrons around the atoms
A chemical compound with the formula HCN is hydrogen cyanide. It is also called prussic acid. It is a liquid that is colorless, highly toxic, and flammable in nature. HCN is a particularly valuable predecessor of numerous chemical compounds ranging from polymers to pharmaceuticals and is processed on an industrial scale.
HCN has a slight bitter almond-like smell due to a recessive genetic mutation that certain individuals are unable to identify. The explosive agent has been used for rodenticide inhalation and human venom, as well as for the slaughter of whales. Cyanide ions interact with respiratory enzymes containing iron as well.
In a variety of plants, this HCN chemical is present in small amounts, particularly in stone fruits such as cherries, as well as in the roots of cassava. Prolonged exposure to a small quantity of cyanide can contribute to chronic health problems in individuals whose diet includes large concentrations of cassava.
Since it prevents cellular oxidative processes, hydrogen cyanide is extremely toxic. Without severe effects, an adult person can tolerate 50 to 60 parts of hydrogen cyanide per million parts of air for an hour, but exposure to concentrations of 200 to 500 parts per million of air for 30 minutes is typically lethal.
Recommended Reading: Why Is Math So Hard For Me
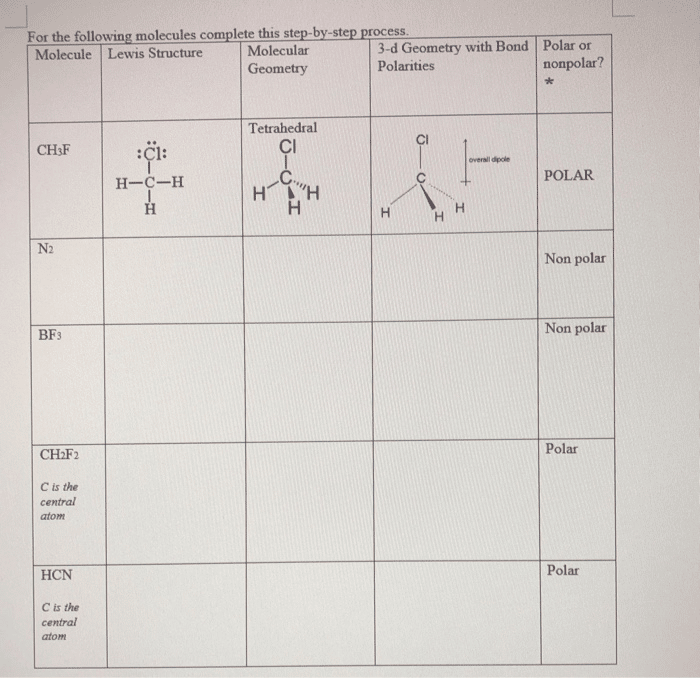
Molecular Geometry Of Ch2f2
The molecular geometry or shape of CH2F2 is tetrahedral. The central atom Carbon is bonded with four atoms and it has. CH2F2 is polar. The central atom is Carbon. It has 4 atoms bonded to it thus, it has a steric number of 4. A steric number of 4 (. CH2F2 has tetrahedral molecular geometry. The central carbon in the compound is sp3 hybridized, which means that one s and p orbitals hybridize to form four sp3. Jul 14, 2021 CH2F2 or Dichloromethane has a quite simple structure as it consists of one central carbon atom forming single covalent bonds with two. The chemical formula CH2F2 represents Difluoromethane. Being a refrigerant, Difluoromethane is also known as HFC-32 or, more commonly, R-32.
Lewis Structure Of Hcn
Some compounds have a very unique and different Lewis structure and HCN is one of those. Thus to understand the Lewis structure in-depth lets go step by step in understanding the concept.
First of all, to remind you Lewiss structure is a pictorial representation of different bonds and lone pair of electrons between two or more atoms of a compound.
Step 1: The foremost step of creating a Lewis structure is finding the valence electrons.
Here we have to find the valence electrons of all three atoms, hydrogen, carbon, and nitrogen.
The number of valence electron is only 1 in Hydrogen because it is an exception atom which doesnt follow the octet rule and thus doesnt need 8 electrons to fill its octet but needs only 1.
Similarly, the valence electrons of Carbon are 4 and that of Nitrogen is 5.
The atomic number of Carbon is 6 so 2 electrons are filled in s orbital and the rest 4 are in the outer orbital that is why the valence number of electrons in carbon is 4.
For Nitrogen, its atomic number is 7, so after 2 electrons occupy s orbital, the rest 5 are in the outer orbital so the valence number of electrons is 5.
Now to find the total number of valence electrons we will add up the valence electrons of all three atoms:
=1+4+5 = 10 valence electrons.
Step 2: Now we will draw the Lewis dot structure of the compound. See the diagram below:
However, hydrogen is the least electronegative but it cant be a central atom because it has only one spare electron.
You May Like: What Is H In Physics
Stage : The Preeminent Advance Of Making A Lewis Structure Is Tracking Down The Valence Electrons
Here we need to track down the valence electrons of each of the three atoms, hydrogen, carbon, and nitrogen.
The quantity of valence electron is just 1 in Hydrogen since it is a special case atom which doesnt keep the octet guideline and in this manner neednt bother with 8 electrons to fill its octet yet needs just 1.
Additionally, the valence electrons of Carbon are 4 and that of Nitrogen is 5.
The atomic number of Carbon is 6 so 2 electrons are filled in s orbital and the rest 4 are in the external orbital for that reason the valence number of electrons in carbon is 4.
For Nitrogen, its atomic number is 7, so after 2 electrons involve s orbital, the rest 5 are in the external orbital so the valence number of electrons is 5.
Presently to observe the complete number of valence electrons we will include the valence electrons of each of the three atoms:
=1+4+5 = 10 valence electrons.
Introduce About Hcn Molecular Geometry & Bond Angles
An explanation of the molecular geometry for the HCN ion including a description of the HCN bond angles. The electron geometry for the Hydrogen cyanide is also provided.
The ideal bond angle for the Hydrogen cyanide is 180° since it has a Linear molecular geometry. Experimentally we would expect the bond angle to be approximately 180°.
To determine the molecular geometry, or shape for a compound like HCN, we complete the following steps:
1) Draw the Lewis Structure for the compound.2) Predict how the atoms and lone pairs will spread out when the repel each other.3) Use a chart based on steric number or use the AXN notation to find the molecular shape. This will be determined by the number of atoms and lone pairs attached to the central atom.If you are trying to find the electron geometry for HCN we would expect it to be Linear.
Helpful Resources:
How to Draw Lewis Structures: Molecular Geometry and VSEPR Explained: Molecular Geo App:Get more chemistry help at
Drawing/writing done in InkScape. Screen capture done with Camtasia Studio 4.0. Done on a Dell Dimension laptop computer with a Wacom digital tablet .
Read Also: Geometry Proofs Examples And Answers
For What Reason Is Hcn Polar
Electronegativity of Hydrogen Cyanide:
Hydrogen wont ever accept a middle situation, as it is the most un-electronegative. Also in light of the distinction in electronegativity among hydrogen and carbon, the vector would be taken from hydrogen to carbon.
Also in a similar way, the vector would move from carbon to nitrogen because of the explanation that nitrogen is more electronegative.
Regardless of just a [minor distinction in the electronegativity of Carbon and Nitrogen, since Nitrogen would endeavor to attract the electrons to itself, it is known as a marginally polar bond.
Furthermore the vector shifts from nitrogen towards hydrogen, since hydrogen has a positive charge and nitrogen, has a negative charge.
Also assuming that any molecule has an electronegativity contrast from the dipoles side it is supposed to be a polar molecule. In this manner, HCN is a polar molecule.
How Many Bond Pairs And Lone Pairs Are There In The Lewis Structure Of Hcn
Technically there are 4 bond pairs and 1 lone pair in the Lewis structure of HCN.
Three bond pairs lie as 1 sigma and 2 pi bonds between the triple bonded C and N atoms while there is a shared bond pair between the single bonded C and H atoms.
There is no lone pair on the central C-atom while 1 lone pair of electrons is present on the N atom.
The three bond pairs between C and N atoms are considered one region of electron density while assigning shape and geometry to the HCN molecule.
Don’t Miss: What Is Biomass In Biology