Even And Odd Nucleon Numbers
| 53 | 286 |
The proton:neutron ratio is not the only factor affecting nuclear stability. It depends also on evenness or oddness of its atomic number Z, neutron number N and, consequently, of their sum, the mass number A. Oddness of both Z and N tends to lower the nuclear binding energy, making odd nuclei, generally, less stable. This remarkable difference of nuclear binding energy between neighbouring nuclei, especially of odd-Aisobars, has important consequences: unstable isotopes with a nonoptimal number of neutrons or protons decay by beta decay , electron capture, or other less common decay modes such as spontaneous fission and cluster decay.
The majority of stable nuclides are even-proton-even-neutron, where all numbers Z, N, and A are even. The odd-A stable nuclides are divided into odd-proton-even-neutron, and even-proton-odd-neutron nuclides. Stable odd-proton-odd-neutron nuclei are the least common.
Even atomic number
The 146 even-proton, even-neutron nuclides comprise ~58% of all stable nuclides and all have spin 0 because of pairing. There are also 24 primordial long-lived even-even nuclides. As a result, each of the 41 even-numbered elements from 2 to 82 has at least one stable isotope, and most of these elements have several primordial isotopes. Half of these even-numbered elements have six or more stable isotopes. The extreme stability of helium-4 due to a double pairing of 2 protons and 2 neutrons prevents any nuclides containing five (5
| 7.04×108a |
Relative Abundance Of Isotopes
The relative abundance of isotopes is the percent of one isotope occurring in nature.
Isotopes of a same atom differ in neutron number, as their proton number remains same. Different isotopes present in different amounts within an element, so it is convenient to find relative abundance of each isotope.
For example, there are two isotopes of chlorine in nature which are 35Cl and 37Cl. To find the average atomic mass of an element, one must find the relative abundance of its isotopes. Mass spectrometer is used to determine relative atomic masses.
Using Isotope Abundance To Calculate Atomic Weight
As stated previously, the number of isotopes and their percent abundance are all that are needed to calculate the atomic weight of an element. We can start by using magnesium as an example. Magnesium has three naturally occurring isotopes: 24Mg, 25Mg, and 26Mg. Each isotope has an abundance of 78.70 %, 10.13%, and 11.17%, respectively. The atomic mass of each isotope is usually very close to each isotope value. In this example, the mass of each isotope is 23.985 amu, 24.985 amu, and 25.982 amu respectively.
Now that we have all of the information about mass and abundance, we can calculate the atomic weight of magnesium. If you have trouble visualizing all of the values, you can organize them in a table to make your information more clear.
| Isotope | |
| 25.982 | 11.17% |
We start by multiplying each isotopes mass by its abundance. This can be done in two ways. First, we can directly multiply the mass by the percent:
23.985 amu = 1887.6 amu
On the other hand, we can change the percent to a decimal out of one and then multiply by the mass. This can be done by dividing the percent by 100.
23.985 amu = 18.876 amu
With both of these methods, the next step is to repeat for the other isotopes and add the values together.
Method 1:
23.985 amu + 24.985 amu + 25.982 amu =
1887.6 amu + 253.09 amu + 290.21 amu = 2430.90 amu
Method 2:
23.985 amu + 24.985 amu + 25.982 amu = 24.3090 amu
2430.90 amu/100= 24.3090 amu
You May Like: What Is Quantitative Techniques In Geography
What Is The Difference Between Percent And Relative Abundance
Home / Asked / What Is The Difference Between Percent And Relative Abundance
Contents
The difference between relative abundance and percent abundance is that relative abundance refers relatively to the number of candies you used in the experiment, where as the Percent abundance is referring to how many of each candy there are in every hundred candies.
What is the difference between relative abundance and percent abundance ?, Percent Abundance vs Relative Abundance
Percent abundance is the percentage amount of all naturally occurring isotopes of an element. Relative abundance of an element is a percentage of the occurrence of an element relative to all other elements in the environment. Percent abundance gives the abundance of isotopes.
Furthermore, What does the relative abundance tell you ?, The relative abundance of an isotope is the percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element. The relative abundance of each isotope can be determined using mass spectrometry.
Finally, What is Percent abundance ?, The relative abundance definition in chemistry is the percentage of a particular isotope that occurs in nature. The atomic mass listed for an element on the periodic table is an average mass of all known isotopes of that element.
What Is Natural Percent Abundance
Natural abundance: The relative amount of the isotopes of an element, as it occurs in nature. Influences intensity of signals in mass spectrometry and NMR spectroscopy . Relative Abundance of Some Isotopes Important in Organic Chemistry. Isotopes not listed are present only in negligible amounts.
Don’t Miss: What Is The Meaning Of Geography
Percent Abundance Vs Relative Abundance
Percent abundance is the percentage amount of all naturally occurring isotopes of an element. Relative abundance of an element is a percentage of the occurrence of an element relative to all other elements in the environment. Representation Percent abundance gives the abundance of isotopes. Relative abundance gives the abundance of chemical elements.
What Is An Isotope
Isotopes are very similar versions of the same element, only having one difference: the number of neutrons. Though these two versions of the same element differ in the number of neutrons, it is important to note that they do not differ in the number of protons and electrons. In some instances, isotopes can have different reactivity, but in most cases, the defining difference is the number of neutrons.
A common example of an isotope having reactivity that differs from what the element is known for is carbon. Carbon is known to be a very stable element, often being involved in predictable reactions. One isotope of carbon, carbon-14, defies the normal reactivity of the stable element. Carbon-14 is a naturally occurring carbon isotope that radioactively decays. Read more about carbon here.
Also Check: What Is Location In Geography
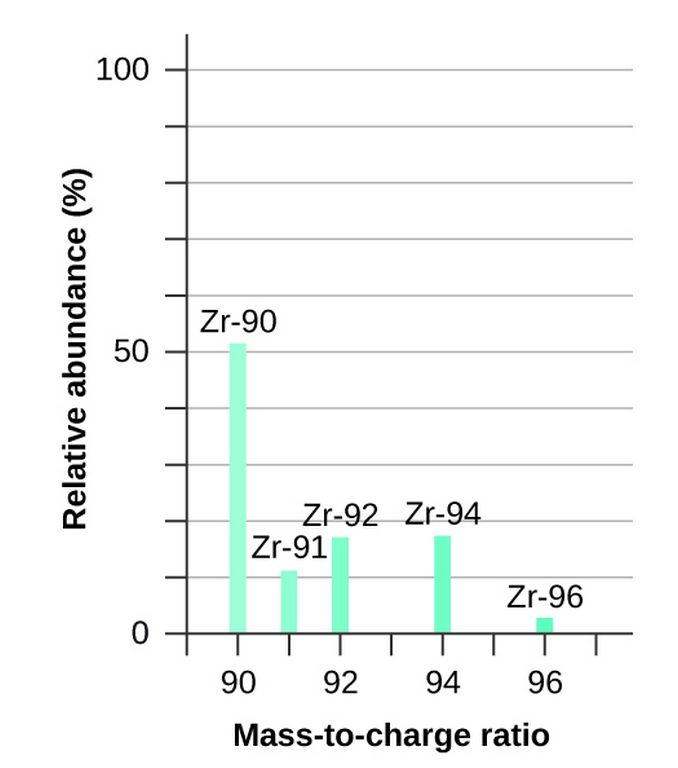
Determination Of Relative Atomic Mass Using Mass Spectrometer
Mass spectrometer allows the measurement of mass to charge ratio from which the relative mass of isotopes is determined. The vapourized sample of chlorine enters an ionizing chamber with high energy electrons. These electrons give enough energy to ionize the chlorine gas by taking off the electron. These ions are then passed through a magnetic field. Under a specific magnetic field, deflection of ions occurs depending upon masses of ions. The heavier ion, moving at slow speed, deflected more than the ion which has lighter mass. This deflection recorded at the detector and the peaks shown on the mass spectrum depicts the relative abundance of isotopes.
Radioactive Primordial And Stable Isotopes
Some isotopes/nuclides are radioactive, and are therefore referred to as radioisotopes or radionuclides, whereas others have never been observed to decay radioactively and are referred to as stable isotopes or stable nuclides. For example, 14C is a radioactive form of carbon, whereas 12C and 13C are stable isotopes. There are about 339 naturally occurring nuclides on Earth, of which 286 are primordial nuclides, meaning that they have existed since the Solar System‘s formation.
Primordial nuclides include 34 nuclides with very long half-lives and 252 that are formally considered as “stable nuclides“, because they have not been observed to decay. In most cases, for obvious reasons, if an element has stable isotopes, those isotopes predominate in the elemental abundance found on Earth and in the Solar System. However, in the cases of three elements the most abundant isotope found in nature is actually one extremely long-lived radioisotope of the element, despite these elements having one or more stable isotopes.
Adding in the radioactive nuclides that have been created artificially, there are 3,339 currently known nuclides. These include 905 nuclides that are either stable or have half-lives longer than 60 minutes. See list of nuclides for details.
Recommended Reading: What Does Polar Mean In Chemistry
Chemical And Molecular Properties
A neutral atom has the same number of electrons as protons. Thus different isotopes of a given element all have the same number of electrons and share a similar electronic structure. Because the chemical behavior of an atom is largely determined by its electronic structure, different isotopes exhibit nearly identical chemical behavior.
The main exception to this is the kinetic isotope effect: due to their larger masses, heavier isotopes tend to react somewhat more slowly than lighter isotopes of the same element. This is most pronounced by far for protium , and tritium , because deuterium has twice the mass of protium and tritium has three times the mass of protium. These mass differences also affect the behavior of their respective chemical bonds, by changing the center of gravity of the atomic systems. However, for heavier elements, the relative mass difference between isotopes is much less so that the mass-difference effects on chemistry are usually negligible. There is also an equilibrium isotope effect.
ZNZNZ
What Is Percent Abundance
The percentage of a specific isotope that exists in nature is the relative concept of percent abundance in chemistry. Abundance can be calculated by three ways: by mass fraction, by mole fraction and by volume fraction. The atomic weight indicated on the periodic table for an element constitutes an average mass of all recognized isotopes.
The atomic nuclei only comprise protons and neutrons, each of these having a mass of around 1 atomic mass unit . The atomic weight of each element should also be a whole number. Moreover, electron weights are considered negligible and are not involved in the atomic weight of an element. As we know that, when we look at the periodic table, it is mentioned that the most of the elements have a decimal fraction of their atomic weights. So, actually, the mass specified for each element is mean of all the isotopes that exist in nature which is. The abundance of each isotope of the element can be determined instantly if we know how often the isotopes have atomic weights.
The recognition of the element appears the same as the number of neutrons inside the nucleus varies. A modification in the number of neutrons in the nucleus indicates an isotope. There might be zero, one, two, more neutrons in the nuclei depending on the element. For example Hydrogen has three isotopes. The nucleus of protium or simply hydrogen consists no neutron but only proton, deuterium has a neutron in the nucleus and tritium has two neutrons in the nucleus.
Don’t Miss: What Is Unit In Physics
Elemental And Isotopic Abundances
The composition of any object can be given as a set of elemental and isotopic abundances. One may speak, for example, of the composition of the ocean, the solar system, or indeed the Galaxy in terms of its respective elemental and isotopic abundances. Formally, the phrase elemental abundances usually connotes the amounts of the elements in an object expressed relative to one particular element selected as the standard for comparison. Isotopic abundances refer to the relative proportions of the stable isotopes of each element. They are most often quoted as atom percentages, as in the table.
While there is general agreement on how the elements formed, the interpretation of elemental and isotopic abundances in specific bodies continues to occupy the attention of scientists. They obtain their raw data from several sources. Most knowledge concerning abundances comes from the study of the Earth, meteorites, and the Sun.
Currently accepted estimates of solar system abundances are pieced together mainly from two sources. Chemical analyses of Type I carbonaceous chondrites, a special kind of meteorite, provide information about all but the most volatile elementsi.e., those that existed as gases that the parent body of the meteorite could not trap in representative amounts. Spectroscopic analysis of light from the Sun furnishes information about the volatile elements deficient in meteorites.
Key Difference Percent Abundance Vs Relative Abundance
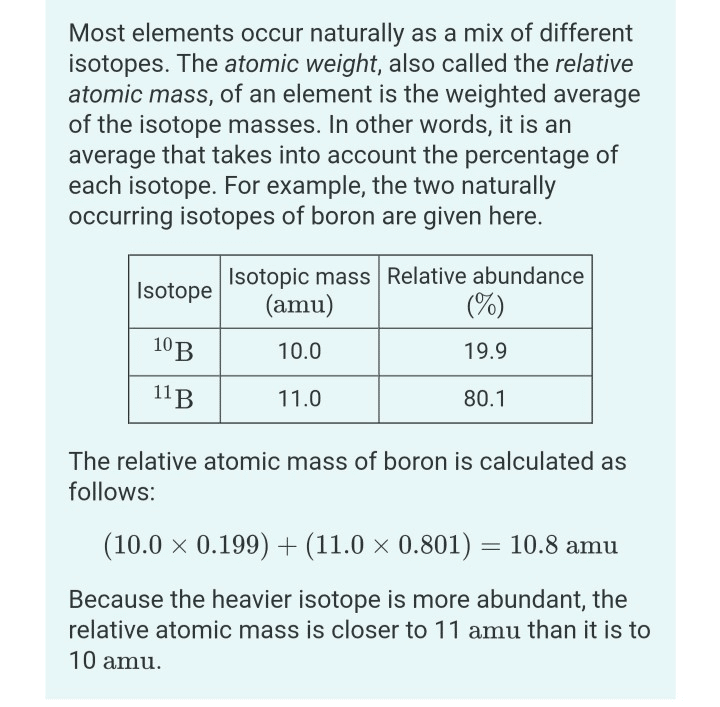
Percent abundance and relative abundance are percentage values of chemical elements that represent their occurrence in the environment. The key difference between percent abundance and relative abundance is that percent abundance gives the abundance of isotopes whereas relative abundance gives the abundance of chemical elements. The percent abundance can be used to determine the average atomic mass of a certain chemical element. Relative abundance gives the occurrence of a certain chemical element in a given environment, i.e, on earth.
You May Like: How Has Japan’s Geography Affected Its History
How To Calculate Percentage Abundance Using Atomic And Isotopic Masses
posted on
Please enable JavaScript
To calculate percentage abundance, you must recall the atomic mass of an element is calculated by using the formula:
Formula for calculating atomic mass
In the above formula you see fractional abundance. How do you get that? To get fractional abundance, you usually divide the percentage abundance of each isotope by 100. And when you add all the fractional abundance values of all the isotopes, you will notice they all add up to 1. To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100. Now, lets apply our understanding to solve the following question:
Silver has two stable isotopes: silver-107 and silver-109 . Silver-107 has a mass of 106.90509 amu and silver-109 has a mass of 108.90476 amu. Calculate the percentage abundance of each isotope.
Strategy
To calculate percentage abundance, we must first know the fractional abundance of each isotope. But from the question, we are not given these values, which means we must think of a way of finding them. One way we can find them is to remember that the:
fractional abundance of isotope 1 plus the fractional abundance of isotope 2 = 1
Setup for calculating fractional abundance
107.8682 amu = 106.90509X amu + 108.90476 amu 108.90476X amu
107.8682 amu 108.90476 amu = 106.90509 X amu 108.90476 X amu
-1.03656
Find The Percent Abundance
To get percent abundance, we will multiply the relative abundance value by 100 and put a percent sign.
As we have now x= 0.76. Simply, multiply by 100 to get percent. So, chlorine-35 is 0.76*100=76%
Now,
= = 0.24
Multiplying above by 100, we get 24%.
Therefore, the percent abundance of chlorine-35 is 76% and the percent abundance of chlorine-37 is 24%.
Recommended Reading: What Is Coplanar In Geometry
What Is Relative Abundance
Isotopes are atoms that have the same number of protons but different numbers of neutrons. The atomic masses of isotopes differ. The percentage of atoms with a specific atomic mass found in a naturally occurring sample of an element is known as its relative abundance.
Mass spectrometry can be used to determine the relative abundance of each isotope.
Since different isotopes have different relative abundances, some are more naturally abundant on Earth than others. Relative abundances are usually expressed as percentages, which means that the relative abundances of all of an elements stable isotopes always add up to 100 percent. The average atomic mass of an element is a weighted average of these values.
Calculating Relative Abundance In Mass Spectroscopy
If a mass spectrum of the element was given, the relative percentage isotope abundances are usually presented as a vertical bar graph. The total may look as if it exceeds 100 percent, but that is because the mass spectrum works with relative percentage isotope abundances.
An example will make this clear. A nitrogen isotope pattern would show a 100 relative abundance for nitrogen-14 and 0.37 for nitrogen-15. To solve this, a ratio such as the following would be set up:
nitrogen-14 = / = 0.996 or 99.6%
nitrogen-15 = / = 0.004 or 0.4%
Related Articles
Don’t Miss: What Does Place Mean In 5 Themes Of Geography
Use The Relative Abundance Concept
Then, we will use the relative abundance formula for the given problem. As the given element consists only two isotopes, an equation can be set by the given equation below
M1x+M21-x=MA
Where,
M1 is the mass of one isotope, x is the relative abundance, M2 is the mass of other isotope and MA is the average atomic mass of the element
As we have, the atomic mass of chlorine-35 is 34.97 and the atomic mass of chlorine-37 is 36.97 amu. So, now lets find the relative abundance.
Here, we need to solve for unknown x which is the relative abundance. One isotope is related as M1 and the other as M2.
We have
Placing the data in the first equation, we get
34.97×x+36.97×1-x=35.45
How Do You Calculate The Percentage Of Abundance Based On Mass
Calculate the average atomic mass using the atomic masses of each isotope and their percent abundances. Divide each percent abundance by 100 to convert it to decimal form. Multiply this value by the isotopes atomic mass. Add the atomic masses of each isotope together to get the average atomic mass.
Recommended Reading: What Does Vf Mean In Physics
Solution For Problem 106p Chapter 4
Introductory Chemistry | 5th Edition
- 2901 Step-by-step solutions solved by professors and subject experts
- Get 24/7 help from StudySoup virtual teaching assistants
Introductory Chemistry | 5th Edition
Copper has two naturally occurring isotopes. Cu-63 has a mass of 62.939 amu and relative abundance of 69.17%. Use the atomic weight of copper to determine the mass of the other copper isotope.
Step 1 of 2
To calculate the atomic weight, the atomic weight of each isotope is multiplied by its percent abundance . Then the results are added together.
i.e. atomic mass = sum of —–
Atomic mass of the element copper = 63.5amu
Atomic mass of first isotope = 62.939amu
Relative abundance = 69.17%
ISBN: 9780321910295
Other solutions