What Is Blackbody Radiation In Chemistry
Asked by: Mrs. Frederique Kassulke
Blackbody radiation is a theoretical concept in quantum mechanics in which a material or substance completely absorbs all frequencies of light. … As the temperature increases, the total radiation emitted also increases due to an increase in the area under the curve.
Major Forms Of Radioactivity
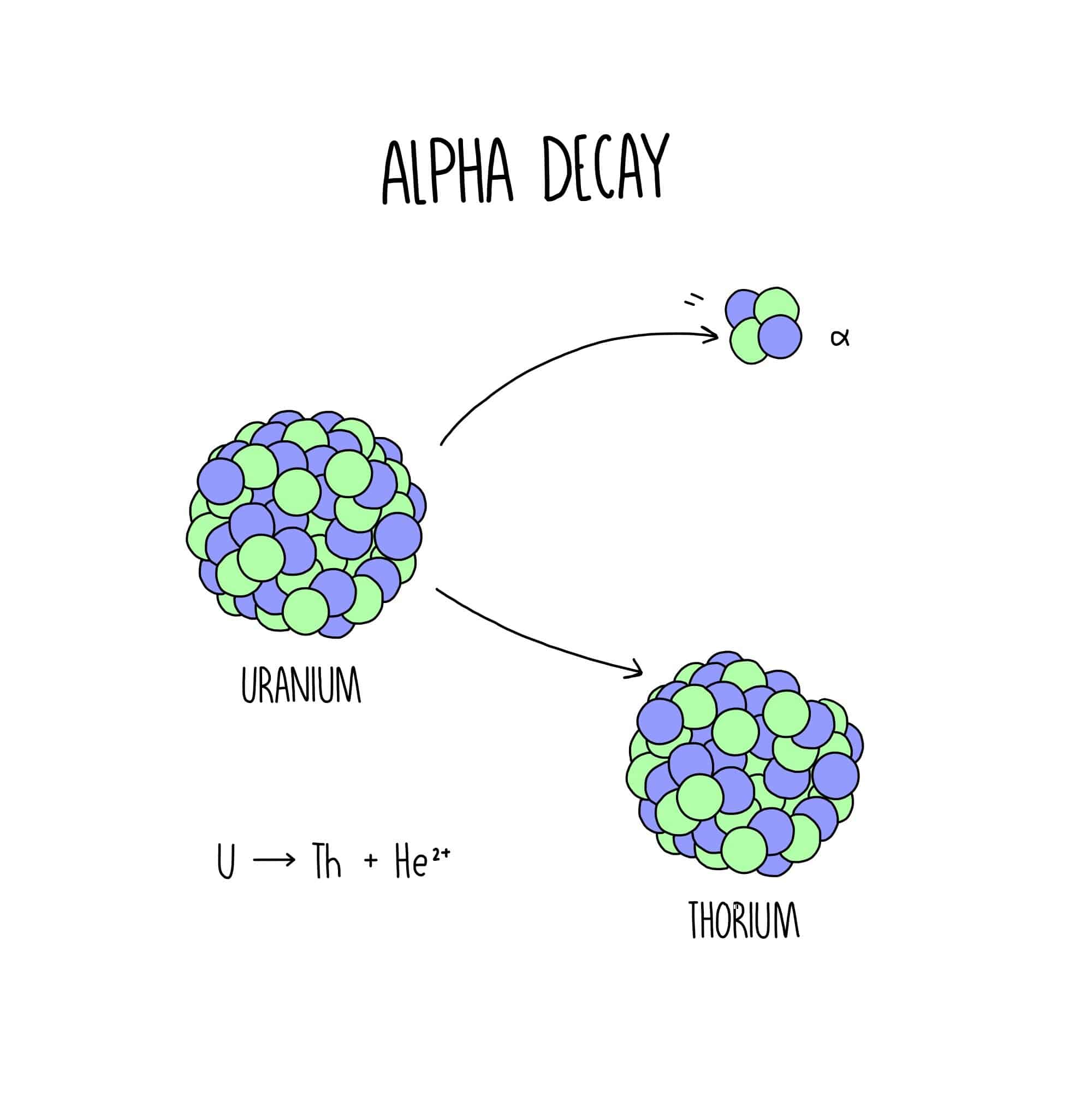
Alpha Particle
Rutherfords experiments demonstrated that there are three main forms of radioactive emissions. The first is called an alpha particle, which is symbolized by the Greek letter . An alpha particle is composed of two protons and two neutrons and is the same as a helium nucleus. It has a 2+ charge. When a radioactive atom emits an alpha particle, the original atoms atomic number decreases by two , and its mass number decreases by four . We can represent the emission of an alpha particle with a chemical equationfor example, the alpha-particle emission of uranium-235 is as follows:
Rather than calling this equation a chemical equation, we call it a nuclear equation to emphasize that the change occurs in an atomic nucleus. How do we know that a product of this reaction is 90231Th? We use the law of conservation of matter, which says that matter cannot be created or destroyed. This means we must have the same number of protons and neutrons on both sides of the nuclear equation. If our uranium nucleus loses 2 protons, there are 90 protons remaining, identifying the element as thorium. Moreover, if we lose four nuclear particles of the original 235, there are 231 remaining. Thus we use subtraction to identify the isotope of the Th atomin this case, 90231Th.
Beta Particle
Again, the sum of the atomic numbers is the same on both sides of the equation, as is the sum of the mass numbers.
Gamma Radiation
Table 3.1 The Three Main Forms of Radioactive Emissions
What Is Radioactivity And Radiation
Radioactivity is the term used to describe the natural process by which some atoms spontaneously disintegrate, emitting both particles and energy as they transform into different, more stable atoms. This process, also called radioactive decay, occurs because unstable isotopes tend to transform into a more stable state. Radioactivity is measured in terms of disintegrations, or decays, per unit time. Common units of radioactivity are the Becquerel, equal to 1 decay per second, and the Curie, equal to 37 billion decays per second.
Radiation refers to the particles or energy released during radioactive decay. The radiation emitted may be in the form of particles, such as neutrons, alpha particles, and beta particles, or waves of pure energy, such as gamma and X-rays.
Each radioactive element, or radionuclide, has a characteristic half-life. Half-life is a measure of the time it takes for one half of the atoms of a particular radionuclide to disintegrate into another nuclear form. Half-lives vary from millionths of a second to billions of years. Because radioactivity is a measure of the rate at which a radionuclide decays , the longer the half-life of a radionuclide, the less radioactive it is for a given mass.
Don’t Miss: What Is The Rdw Process In Math
The Nature Of Radioactive Emissions
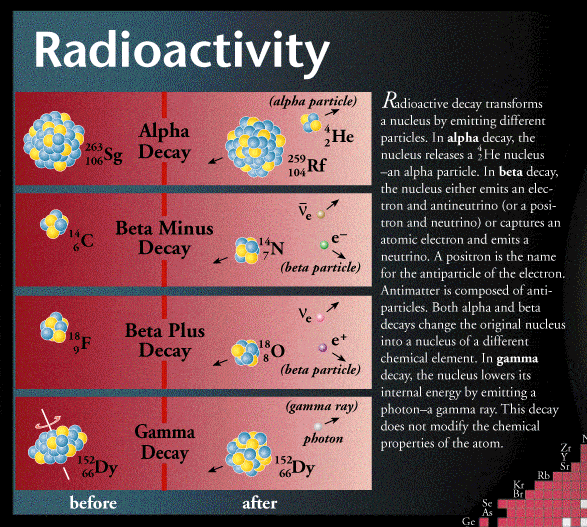
The emissions of the most common forms of spontaneous radioactive decay are the alpha particle, the beta particle, the gamma ray, and the neutrino. The alpha particle is actually the nucleus of a helium-4 atom, with two positive charges 4/2He. Such charged atoms are called ions. The neutral helium atom has two electrons outside its nucleus balancing these two charges. Beta particles may be negatively charged , or positively charged . The beta minus particle is actually an electron created in the nucleus during beta decay without any relationship to the orbital electron cloud of the atom. The beta plus particle, also called the positron, is the antiparticle of the electron when brought together, two such particles will mutually annihilate each other. Gamma rays are electromagnetic radiations such as radio waves, light, and X-rays. Beta radioactivity also produces the neutrino and antineutrino, particles that have no charge and very little mass, symbolized by and , respectively.
Calculation Of Radioactive Decay
It is important for the healthcareprofessional to predict the activity of the radioactive material at any pointin time before or after the assay being undertaken, as it is crucial to knowthe exact activity at administration to the patient. The radioactive decay canbe described as the average number of radioactive isotopes disin-tegrating per unit time. The disintegration rate is defined as dN/dt.The disintegration rate is proportional to the number of undisposedradioisotopes, and can be also expressed as the activity .
Upon integration, the radioactive decay of any radioactivesample can be calculated by applying the so-called radionuclide decay equation. In order to calculate the radioactivity at a specific timepoint t, it is important to know theinitial activity A0, theelapsed time t and the decay constant. Half-life is the time that passesby until the activity has halved.
Example
Aradioactive sample has a half-life of 8.05 days and contains 150 mCiradioactivity. Calculate the radioac-tivity left after 20 days.
Recommended Reading: What Is Compliance In Psychology
The Structure Of An Atom And Radioactivity
Itâs actually due to radioactivity that we even understand the underlying structure of the atom at all. After the discovery of the electron in 1897 by J. J. Thomson, the most popular theory of how an atom was structured was the plum pudding model or the Thomson Model. Thomson proposed that negatively chargedâplumsâ were surrounded by a positively chargedâpuddingâ.
Scattering of alpha particles if Plum Pudding model was correct compared to the real results, commons.wikimedia
In 1905, Ernst Rutherford tested the plum pudding model by directing a beam of alpha particles at a strip of gold foil. Alpha particles are a form of radiation with a large positive charge. He expected the alpha particles to pass through the gold with no deflection as the positively charged âpuddingâ should be evenly spread out. However, a very small number of the alpha particles were deflected, sometimes being reflected completely.
He proposed that the atom actually consisted of a small, compact, and positively charged nucleus surrounded by a cloud of electrons, called the Rutherford model. The vast majority of the alpha particles passed through the atom without any deflection, proving how small the nucleus was compared to the atom as a whole.
What Is Radioactivity Explain
Radioactivity is the term used to describe the natural process by which some atoms spontaneously disintegrate, emitting both particles and energy as they transform into different, more stable atoms. This process, also called radioactive decay, occurs because unstable isotopes tend to transform into a more stable state.
You May Like: What Does Evapotranspiration Mean In Geography
What Is Radioactivity In Chemistry For Kids
Radioactivity is simply when very small particles in objects emit energy or smaller particles. The energy that is produced can result in cancer, serious environmental damage, or helpful technologies. There are different degrees of radioactivity, and different exposures increase the harm it can cause.
Types Of Radioactive Decay
The first three types of radioactive decay to be discovered were alpha, beta, and gamma decay. These modes of decay were named by their ability to penetrate matter. Alpha decay penetrates the shortest distance, while gamma decay penetrates the greatest distance. Eventually, the processes involved in alpha, beta, and gamma decay were better understood and additional types of decay were discovered.
- Alpha decay: An alpha particle is emitted from the nucleus, resulting in a daughter nucleus .
- Proton emission: The parent nucleus emits a proton, resulting in a daughter nucleus .
- Neutron emission: The parent nucleus ejects a neutron, resulting in a daughter nucleus .
- Spontaneous fission: An unstable nucleus disintegrates into two or more small nuclei.
- Beta minus decay: A nucleus emits an electron and electron antineutrino to yield a daughter with A, Z + 1.
- Beta plus decay: A nucleus emits a positron and electron neutrino to yield a daughter with A, Z – 1.
- Electron capture: A nucleus captures an electron and emits a neutrino, resulting in a daughter that is unstable and excited.
- Isomeric transition : An excited nucleus releases a gamma ray resulting in a daughter with the same atomic mass and atomic number ,
Gamma decay typically occurs following another form of decay, such as alpha or beta decay. When a nucleus is left in an excited state it may release a gamma ray photon in order for the atom to return to a lower and more stable energy state.
Don’t Miss: What Is Conjugation In Biology
Occurrence Of Alpha Decay
Alpha decay occurs only in the heaviest of the elements. The elements nucleus should be large or unstable enough to undergo spontaneous fission-type changes. It is the most common form of decay in such elements. The alpha particles emitted out of the nucleus usually have an energy level of around 5 MeV and have a speed of around 5% of light. It is important to note that alpha particles possess a charge of +2 due to the absence of electrons. Due to this charge and owing to its heavy mass, an alpha particle reacts with the surroundings vigorously to lose all of its energy almost immediately. Their forward motion can be stopped by a few centimeters of air.
Owing to their heaviness and their charge, this kind of radioactive decay reacts most violently with the human body. They have a high ionizing power due to which they can wreak havoc with a tissue. An overdose of alpha radiation results in the formation of blisters and burns on the victims bodies.
Key Takeaways: Definition Of Radioactivity
- Radioactivity is the process by which an unstable atomic nucleus loses energy by emitting radiation.
- While radioactivity results in the release of radiation, not all radiation is produced by radioactive material.
- The SI unit of radioactivity is the becquerel . Other units include the curie, gray, and sievert.
- Alpha, beta, and gamma decay are three common processes through which radioactive materials lose energy.
Also Check: What Is Distillation In Chemistry
Inature Notation And Units
Radioactivity is the phenomenon of the spontaneous disintegration of unstable atomic nuclei to atomic nuclei to form more energetically stable atomic nuclei. Radioactive decay is a highly exoergic, statistically random, first-order process that occurs with a small amount of mass being converted to energy. Since it is a first-order process, each radioactive species is characterized by its own half-life, the length of time in which an initially very large number of such nuclei will have decayed to only half the original number. In radioactive decay, a relatively large amount of energy is liberated in each disintegrationtypically about 1 million times more than the amount of energy liberated in an exothermic chemical reaction, that is, a few million electron volts of energy per nucleus, compared to only a few electron volts of energy per atom or molecule. Since radioactive decay is a nuclear rather than an electronic phenomenon, its rate for a given radioactive species is not altered measurably by changes in temperature or pressure the only exception to this is the production of very slight changes in half-life by the use of great pressures on a few radionuclides that decay by the process of orbital electron capture .
W. Greiner, D.N. Poenaru, in, 2005
Read A Brief Summary Of This Topic
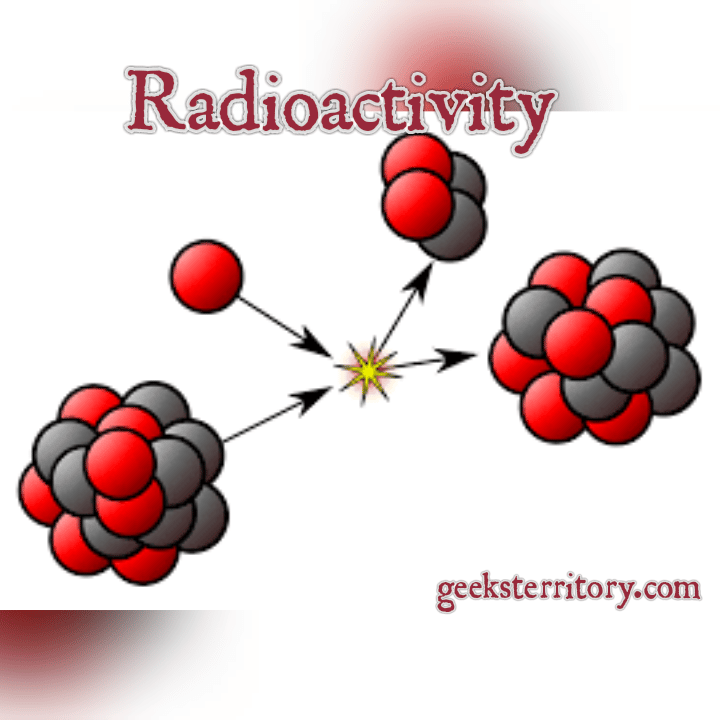
radioactivity, property exhibited by certain types of matter of emittingenergy and subatomic particles spontaneously. It is, in essence, an attribute of individual atomic nuclei.
An unstable nucleus will decompose spontaneously, or decay, into a more stable configuration but will do so only in a few specific ways by emitting certain particles or certain forms of electromagnetic energy. Radioactive is a property of several naturally occurring elements as well as of artificially produced isotopes of the elements. The rate at which a radioactive element decays is expressed in terms of its half-life i.e., the time required for one-half of any given quantity of the isotope to decay. Half-lives range from more than 1024 years for some nuclei to less than 1023 second . The product of a radioactive processcalled the daughter of the parent isotopemay itself be unstable, in which case it, too, will decay. The process continues until a stable nuclide has been formed.
You May Like: What Was The First School Of Thought In Psychology
Atoms And Radioactivity: Beta Particle
Oppositely to alpha decay, if an unstable nucleus has too many neutrons compared to protons, it will emit a beta âβâ particle. A neutron within the nucleus will spontaneously turn into a proton, ejecting a high-velocity electron in the process. The beta particle is literally just one electron.
A Caesium-137 nucleus decays into Barium-137 and emits a beta particle, commons.wikimedia
Beta decay will cause an atom to change to a different element. Remember that a neutron has been converted into a proton. This will increase the proton number of the nucleus by one but keep the mass number unchanged, as an electron has virtually no mass. A beta particle can be written asorin the context of nuclear equations. The nuclear equation of beta decay of Caesium-137 into Barium-137 shown in the example above is.
Atoms And Radioactivity: Neutron Emission
Some radioactive isotopes are capable of decay by emitting neutrons âηâ at high velocities. It is most commonly seen during nuclear fission of high mass radioactive isotopes with a high neutron to proton ratio. Depending on the isotope that is undergoing decay, one or multiple neutrons can be emitted at once.
Neutron emission during the fission of an atomic nucleus, flickr
When a nucleus emits a neutron, its mass number decreases by 1, but its proton number remains the same. It is generally written as. An atomâs designated element depends only on the proton number and not the mass number. This means that neutron emission alone will never change the element of an atom, although it will change it to a different isotope.
You May Like: What Does Plane Mean In Geometry
Advantages And Disadvantages Of Radioactivity
Advantages of radioactivity are:
- Gamma rays are used to kill cancerous cells and hence used in radiotherapy.
- Cobalt-60 is used to destroy carcinogenic cells.
- Gamma rays are used in scanning the internal parts of the body.
- Gamma rays kill microbes present in food and prevent it from decay by increasing the shelf life.
- Age of the rocks can be studied using radioactive radiations by measuring the argon content present in the rock.
Disadvantages of radioactivity are:
- High dosage of radioactive radiation on the body might lead to death.
- Radioactive isotopes are expensive.
In this article, you learned what is radioactivity. Want to know more? Join BYJUS and fall in love with learning. Also, register to BYJUS The Learning App for loads of interactive, engaging Physics-related videos and unlimited academic assist.
Radioactive Isotopes Of Carbon
Carbon-12 ) and carbon-13 ) are both considered stable isotopes of carbon. However, there are some isotopes of carbon that are considered unstable, and and therefore radioactive.
Carbon-14 is a radioactive isotope of carbon ). It has 6 protons and 8 neutrons and will most likely undergo beta decay to decay into a stable isotope: nitrogen-14.
$$ ^_\text\longrightarrow \text^_\text^_\text $$
Don’t Miss: What Does The Word Biology Mean
The Effects Of Radioactivity On An Atom
A radioactive atom will be changed after undergoing radioactive decay, which can happen in several different ways. Radioactive decay can occur due to an unstable nucleus emitting radiation. The most common forms of decay are alpha particles, beta particles, gamma-rays, or neutron emissions. Each type of radiation has different properties and characteristics.
What Is Radioactive In Physics
Radioactivity is the phenomenon of the spontaneous disintegration of unstable atomic nuclei to atomic nuclei to form more energetically stable atomic nuclei. Radioactive decay is a highly exoergic, statistically random, first-order process that occurs with a small amount of mass being converted to energy.
Don’t Miss: What Does Volume Mean In Math
What Is A Radioactive Decay Chain
U-238 emits an alpha
Thorium 234 emits a beta
Protactinium 234 emits a beta
Uranium 234 emits an alpha
Thorium 230 emits an alpha
Radium 226 emits an alpha
Radon 222 emits an alpha
Polonium 218 emits an alpha
Lead 214 emits a beta
Bismuth 214 emits a beta
Polonium 214 emits an alpha
Lead 210 emits a beta
Bismuth 210 emits a beta
Polonium 210 emits an alpha
Lead 206, which is stable
Atoms And Radioactivity: Alpha Radiation
When the nucleus of an atom has too few neutrons compared to protons, it will emit an alpha particle âαâ, which is made from twoprotons and two neutrons. This helps to restore the balance within the nucleus and reduce the ratio of protons to neutrons.
An Americium-241 nucleus decays into Neptunium-237 and emits an alpha particle, commons.wikimedia
An alpha particleis exactly the same as a helium nucleus. Therefore, alpha decay will cause the nucleus of an atom to lose a mass number of 4 and a proton number of 2. This is helpful when using nuclear equations, as we are able to determine what element the nucleus will decay into.
A radium nucleus emits an alpha particle. What element has the radium nucleus decayed into?
Refer to the periodic table. Radium has a proton number of 88 and a mass number of 226:
One helium nucleus is emitted in alpha decay, so subtract 4 from the mass number and 2 from the proton number of radium:
Determine which element has a proton number of 86 on the periodic table. The answer is Radon, .
Don’t Miss: What Does Pt Stand For In Math
What Is Radioactivity And Its Unit
What is the SI Unit of Radioactivity? The SI unit of radioactivity is becquerel and this term is named after Henri Becquerel. Unit of radioactivity is defined as: The activity of a quantity of radioactive material where one decay takes place per second. 1 becquerel = 1 radioactive decay per second = 2.703×10-11 Ci.