Radioactivity And Nuclear Chemistry
Atomic theory in the nineteenth century presumed that nuclei had fixed compositions. But in 1896, the French scientist Henri Becquerel found that a uranium compound placed near a photographic plate made an image on the plate, even if the compound was wrapped in black cloth. He reasoned that the uranium compound was emitting some kind of radiation that passed through the cloth to expose the photographic plate. Further investigations showed that the radiation was a combination of particles and electromagnetic rays, with its ultimate source being the atomic nucleus. These emanations were ultimately called, collectively, radioactivity.
Following the somewhat serendipitous discovery of radioactivity by Becquerel, many prominent scientists began to investigate this new, intriguing phenomenon. Among them were Marie Curie , who was the first to coin the term radioactivity, and Ernest Rutherford , who investigated and named three of the most common types of radiation. During the beginning of the twentieth century, many radioactive substances were discovered, the properties of radiation were investigated and quantified, and a solid understanding of radiation and nuclear decay was developed.
Figure 3.1 A nucleus of uranium-238 undergoes decay to form thorium-234 . The alpha particle removes two protons and two neutrons from the uranium-238 nucleus.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
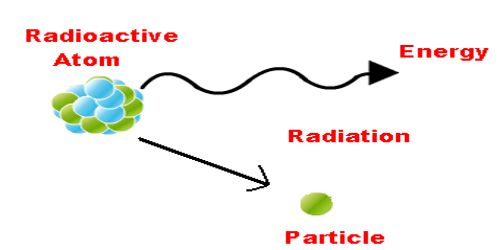
What Is Radiation And How Does It Differ From Radioactivity
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Radiation and radioactivity are two easily confused concepts. Just remember, a substance does not need to be radioactive to emit radiation. Let’s look at the definition of radiation and see how it differs from radioactivity.
Also Check: Elastic Force Definition Physics
Sterilisation Of Aqueous Solutions Of Proteins And Enzymes: Reactions Of Free Radicals With Proteins
The radiation chemistry of amino acids, peptides, proteins and enzymes has been the subject of several reviews . As can be expected, these demonstrate both the complexity of free radical-induced chemistry and the diversity of protein structure, content and conformation. A review of protein radiation chemistry as it applies to the sterilisation of healthcare products containing proteins and enzymes has also been made by Parsons . It is not the purpose here, therefore, to focus on the radiation chemistry. Instead, the focus is placed on those studies carried out at high doses applicable to sterilisation and, in particular, those methods used to protect these sensitive biomolecules against ionising radiation. A brief outline of the major reaction pathways for the primary free radicals of water radiolysis will, however, be useful to place such studies in context, as follows.
The rates of reaction of the hydrated electron with aromatic amino acids are approximately a factor of ten lower than those measured for histidine, cysteine and cystine . Combined with the high reactivity of the peptide bond with eaq,this fact indicates that reactions of the latter with proteins will be dominated by its reactions with the peptide bond, with the protonated histidine residue and with cysteine and cystine.
B. PARSONS, in, 2010
Radiation Interactions With Matter
As ionizing radiation moves through matter its energy is deposited through interactions with the electrons of the absorber. The result of an interaction between the radiation and the absorbing species is removal of an electron from an atom or molecular bond to form radicals and excited species. The radical species then proceed to react with each other or with other molecules in their vicinity. It is the reactions of the radical species that are responsible for the changes observed following irradiation of a chemical system.
Charged radiation species interact through Coulombic forces between the charges of the electrons in the absorbing medium and the charged radiation particle. These interactions occur continuously along the path of the incident particle until the kinetic energy of the particle is sufficiently depleted. Uncharged species undergo a single event per photon, totally consuming the energy of the photon and leading to the ejection of an electron from a single atom. Electrons with sufficient energy proceed to interact with the absorbing medium identically to radiation.
Don’t Miss: Define Movement Geography
What Causes Atoms To Be Radioactive
Atoms found in nature are either stable or unstable. An atom is stable if the forces among the particles that makeup the nucleus are balanced. An atom is unstable if these forces are unbalanced if the nucleus has an excess of internal energy. Instability of an atom’s nucleus may result from an excess of either neutrons or protons. A radioactive atom will attempt to reach stability by ejecting nucleons , as well as other particles, or by releasing energy in other forms.
The band of nuclear stability indicates various neutron/proton combinations that give rise to observable nuclei with measurable half-lives. A close-up look at the band of nuclear stability in the region from Z = 66 through Z = 79 shows the types of radioactive processes undergone by various nuclides. Nuclides with lower neutron/proton ratios tend to undergo positron emission, electron capture, or alpha emission, whereas nuclides with higher neutron/proton ratios tend to undergo beta emission.
Biological Effects Of Radiation Exposure
There is a large difference in the magnitude of the biological effects of nonionizing radiation and ionizing radiation, emissions energetic enough to knock electrons out of molecules .
Figure 3.6.Damaging Effects of Ionizing Radiation. Lower frequency, lower-energy electromagnetic radiation is nonionizing, and higher frequency, higher-energy electromagnetic radiation is ionizing.
Energy absorbed from nonionizing radiation speeds up the movement of atoms and molecules, which is equivalent to heating the sample. Although biological systems are sensitive to heat , a large amount of nonionizing radiation is necessary before dangerous levels are reached. Ionizing radiation, however, may cause much more severe damage by breaking bonds or removing electrons in biological molecules, disrupting their structure and function .
Figure 3.7. Biological Effects of Ionizing Radiation. Ionizing radiation can directly damage a biomolecule by ionizing it or breaking its bonds
Radiation can harm either the whole body or eggs and sperm . Its effects are more pronounced in cells that reproduce rapidly, such as the stomach lining, hair follicles, bone marrow, and embryos. This is why patients undergoing radiation therapy often feel nauseous or sick to their stomach, lose hair, have bone aches, and so on, and why particular care must be taken when undergoing radiation therapy during pregnancy.
Recommended Reading: What Are The Major Specialties In Psychology
Radiation Chemistry Applied In Industrial Processing Equipment
To process materials, either a gamma source or an electron beam can be used. The international type IV irradiator is a common design, of which the JS6300 and JS6500 gamma sterilizers are typical examples. In these irradiation plants, the source is stored in a deep well filled with water when not in use. When the source is required, it is moved by a steel wire to the irradiation room where the products which are to be treated are present these objects are placed inside boxes which are moved through the room by an automatic mechanism. By moving the boxes from one point to another, the contents are given a uniform dose. After treatment, the product is moved by the automatic mechanism out of the room. The irradiation room has very thick concrete walls to prevent gamma rays from escaping. The source consists of 60Co rods sealed within two layers of stainless steel. The rods are combined with inert dummy rods to form a rack with a total activity of about 12.6PBq .
Other Molecules Of Interest
Nitrogen dioxide, when added at levels as low as 0.5%, increased the yield of CO by reacting with O and C radicals to produce CO and NO . This scavenging reactions of C and O with NO2 prevent the backward recombination reactions to produce CO2. Thus, NO2 is considered an inhibitor in these reactions.
The apparent stability of CO2 under high-energy radiation, in the presence of sulfur dioxide and nitrogen dioxide was evidenced by the measured G value for CO production. Both oxides, SO2 and NO2 act as scavengers rendering a G = 3.51 for -radiation, within a wide range of additives, partial pressures, and radiation doses . The reported G value are shown to be in agreement with the value measured by a different -radiation source within experimental error of the dosimetry measurement method. Transient IR spectroscopy combined with pulse radiolysis has resulted in the first IR detection of CO – in acetonitrile with an asymmetric stretch at 1,650 cm1 . The properties and reactivity of CO – in acetonitrile are of interest in understanding electrochemical CO2 reduction in aprotic solvents. Recombination reactions with solvent-derived radicals, in neat CH3CN are responsible for the rapid decay of the anion radicals , while the addition of formate substantially increased the radiation yield of the anion radicals.
Recommended Reading: Eric Van Wilderman Geometry Dash
Reduction Of Metal Compounds
In addition to the reduction of organic compounds by the solvated electrons it has been reported that upon irradiation a pertechnetate solution at pH 4.1 is converted to a colloid of technetium dioxide. Irradiation of a solution at pH 1.8 soluble Tc complexes are formed. Irradiation of a solution at pH 2.7 forms a mixture of the colloid and the soluble Tc compounds. Gamma irradiation has been used in the synthesis of nanoparticles of gold on iron oxide .
It has been shown that the irradiation of aqueous solutions of lead compounds leads to the formation of elemental lead. When an inorganic solid such as bentonite and sodium formate are present then the lead is removed from the aqueous solution.
Sources Of Further Information And Advice
To gain a good insight into the mechanistic aspects of the radiation chemistry and biochemistry of cells, viruses and their components, The Chemical Basis of Radiation Biology by von Sonntag is recommended . For guidance on standards to be adopted for the sterilisation of healthcare and tissue allografts, the ISO and IAEA references contained in this chapter are essential reading. Finally, the websites of the International Irradiation Association and of the International Atomic Energy Agency are very useful to keep abreast of current practices in sterilisation and for international meetings relevant to sterilisation by ionising radiation.
P. Forster, in, 2003
Recommended Reading: What Is The Molecular Geometry Of Ccl4
What Is Electromagnetic Radiation
Whenever a charge is placed in an electric or a magnetic field, it experiences a certain force acting on it or if multiple charges are placed, they experience an interaction due to another.
In the year 1870, James Maxwell became the first scientist to explain the interaction between the charges in the presence of the electric and magnetic fields. He proposed that when electrically charged particles perform an accelerating motion, alternating electrical and magnetic fields are produced and transmitted. These fields traverse in the forms of waves known as electromagnetic radiation. A light wave is an example of electromagnetic radiation.
Theory Of Radiation Chemistry Ii Track Effects In Radiolysis Of Water
21
Section:
You May Like: Who Is The Biological Father Of Elton John’s Sons
The Band Of Stability
Carbon-12, with six protons and six neutrons, is a stable nucleus, meaning that it does not spontaneously emit radioactivity. Carbon-14, with six protons and eight neutrons, is unstable and naturally radioactive. Among atoms with lower atomic numbers, the ideal ratio of neutrons to protons is approximately 1:1. As the atomic number increases, the stable neutron-proton ratio gradually increases to about 1.5:1 for the heaviest known elements. For example, lead-206 is a stable nucleus that contains 124 neutrons and 82 protons, a ratio of 1.51 to 1.
This observation is shown in the figure below. The band of stability is the range of stable nuclei on a graph that plots the number of neutrons in a nuclide against the number of protons. Known stable nuclides are shown with individual blue dots, while the 1:1 and 1.5:1 ratios are shown with a solid red line and a green line, respectively.
It should be noted that just because a nucleus is “unstable” does not mean that it will rapidly decompose. For example, uranium-238 is unstable because it spontaneously decays over time, but if a sample of uranium-238 is allowed to sit for 1000 years, only \ of the sample will have decayed. However, other unstable nuclei, such as berkelium-243, will be almost completely gone decayed) in less than a day.
Is Nuclear Chemistry Good Or Bad
The truth is this: Nuclear chemistry, like anything else, is a technology that can do both great and terrible things. For example, nuclear processes have helped to make enough power to keep society running, and have also allowed the long-range space probes to continue operating for over thirty years.
Read Also: Figure And Ground Psychology
Summary Of Nuclear Radiation
The table below summarizes the main types of nuclear radiation, including charge, mass, symbol, and penetrating power. Penetrating power refers to the relative ability of the radiation to pass through common materials. Radiation with high penetrating power is potentially more dangerous because it can pass through skin and do cellular damage.
Summary of types of nuclear radiation.
| Type |
|---|
What Happens To Atoms After They Release Radiation
As the nucleus emits radiation or disintegrates, the radioactive atom transforms to a different nuclide. This process is called radioactive decay. It will continue until the forces in the nucleus are balanced. For example, as a radionuclide decays, it will become a different isotope of the same element if it gives off neutrons or a different element altogether if it gives off protons.
The series of transformations that a radionuclide goes through to reach stability and the type of radiation produced is characteristic of the radionuclide. The stages form a decay series.
You May Like: Does Elton John Have Biological Children
What Is Electromagnetic Spectrum In Chemistry
electromagnetic spectrumelectromagnetic radiationelectromagnetic spectrumelectromagnetic radiation
. Similarly, you may ask, what is the electromagnetic spectrum simple definition?
Definition of electromagnetic spectrum. : the entire range of wavelengths or frequencies of electromagnetic radiation extending from gamma rays to the longest radio waves and including visible light.
Similarly, what is electromagnetic radiation in chemistry? Electromagnetic radiation is a form of energy that is produced by oscillating electric and magnetic disturbance, or by the movement of electrically charged particles traveling through a vacuum or matter.
Also, how do you explain the electromagnetic spectrum?
The electromagnetic spectrum is a continuum of all electromagnetic waves arranged according to frequency and wavelength. The sun, earth, and other bodies radiate electromagnetic energy of varying wavelengths. Electromagnetic energy passes through space at the speed of light in the form of sinusoidal waves.
What is the electromagnetic spectrum used for?
Nearly all frequencies and wavelengths of electromagnetic radiation can be used for spectroscopy. Radio waves, infrared rays, visible light, ultraviolet rays, X-rays, and gamma rays are all types of electromagnetic radiation. Radio waves have the longest wavelength, and gamma rays have the shortest wavelength.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Read Also: Elastic Force Physics