Measuring Enthalpy Of Reaction Experimentally
An expanded polystyrene cup can be used as a calorimeter as shown in the diagram on the right because polystyrene foam is a good insulator and will prevent heat from the reaction being lost, or, heat from the surroundings entering the reaction mixture.1
How To Calculate The Temperature From Heat Of Neutralisation
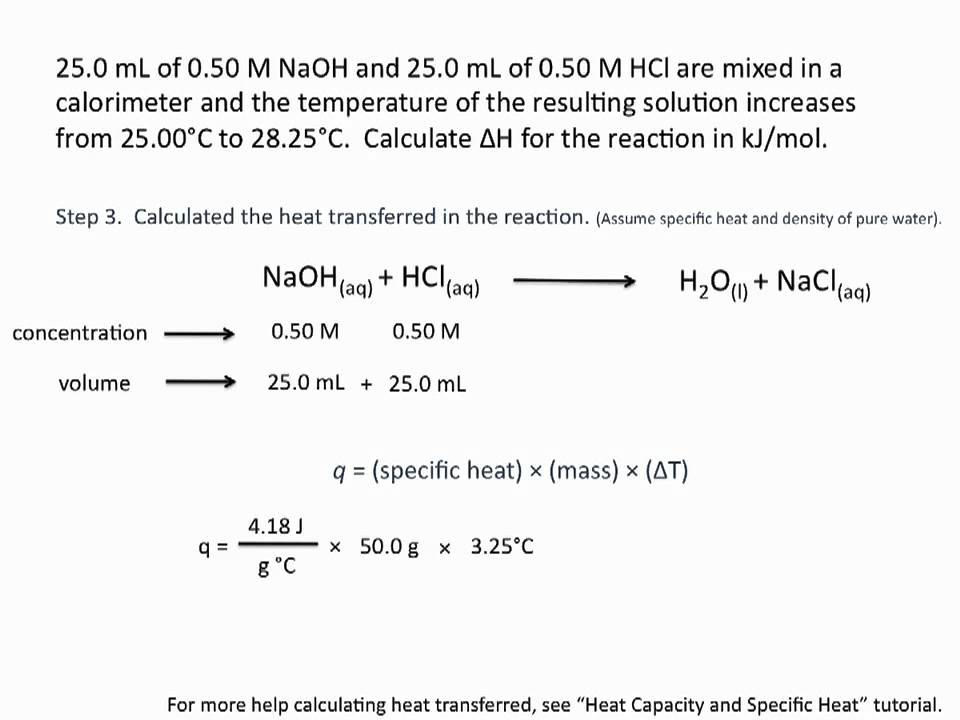
The heat of neutralization of $\ce$ by $\ce$ is $\pu$$\ce$ produced. If $\pu$ of $\pu$$\ce$ at $\pu$ is added to $\pu$ of $\pu$$\ce$ at $\pu$ in a plastic foam cup calorimeter, what will be solution temperature be immediately after the neutralization reaction has occurred?
I’m always overwhelmed by the question if it provided me too much information. I don’t know what equation and concept to use first.
I need a detailed explanation to understand! I’m not sure which one is the initial temperature? $\pu$ of $\ce$ or $\pu~\ce$?
First, you need to recognize what the limiting reagent is. Because NaOH and HCl react in a 1:1 ratio, and there is less HCl , HCl is the limiting reagent.
Second, given the amount of limiting reagent, how much heat will be released . $\pu \times \pu \times \pu$
Third, you need to approximate that the solution has the heat capacity of water, which is $\pu$.
If you mix two volumes of the same substance at different temperatures, the temperature of combined volumes will be approximately the volume-weighted average. So here: $$/75 = 25.55$$
So finally calculate the amount the temperature of $\pu$ of “water” will increase when $\pu \times \pu \times \pu$ of heat is added, and add this to $\pu$.
Heat Of Formation Table For Common Compounds
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Also, called standard enthalpy of formation, the molar heat of formation of a compound is equal to its enthalpy change when one mole of a compound is formed at 25 degrees Celsius and one atom from elements in their stable form. You need to know the values of the heat of formation to calculate enthalpy, as well as for other thermochemistry problems.
This is a table of the heats of formation for a variety of common compounds. As you can see, most heats of formation are negative quantities, which implies that the formation of a compound from its elements is usually an exothermic process.
You May Like: Geometry Basics Segment Addition Postulate Worksheet Answer Key
Calculation On Heat Of Neutralization
1. -57.4KJmol-1 of heat was given off when 1 mole of hydrogen ion of a strong acid neutralizes 1 mole of a hydroxyl of a strong base. Calculate the heat evolved when:
a. 2 mole of H+
b. 0.5 mole of H+ reacts with excess alkaline in dilute solution
c. Calculate the number of moles of H+ that would be produced by the liberation of -5.7KJmol-1
H+ + OH H2O H = -57.4KJmol-1
a. 1 mole of H+ neutralizes 1 mole of OH
= 1 x -57.4 = -57.4KJmol-1 . Therefore when 2 moles of H+ neutralizes 2 moles of OH-, = 2 x -57.4 = -114.8KJmol-1 of heat was liberated.
b. 0.5 mole of H+ neutralizes 0.5KJmol-1 of OH = -57.5 x 0.5 = -28.7KJmol-1 of heat was liberated.
c. -57.4KJmol-1 of heat gives 1 mole of H+, therefore -57.4KJmol-1 will give
-57.4/-57.4 x 1/1
= 0.1 mole of H+
Measuring Heat Of Combustion By Temperature Change
Suppose you have been asked to calculate the heat of combustion evolved from the burning of 1.75g ethanol along with 200g of water. During this reaction, the temperature of the system has been observed to increase up to 55 degrees centigrade. To calculate the molar heat of combustion of 1.75g ethanol, follow this scheme
Also Check: What Does K Stand For In Math
Solved Examples For Heat Of Reaction Formula
Q.1: Determine the heat change using Heat of Reaction Formula which accompanies the combustion of a chemical when a certain mass of the substance is burnt in air to raise the temperature of 200 g of water initially at \. Specific heat capacity of water is given as \
Solution: Given parameters in the problem are as below,
- m = 200 g
- \
- \
- \
According to this problem, a certain mass of ethanol is burnt to raise the temperature of the water. It means heat absorbed by water is evolved from the combustion reaction of ethanol.
Since heat loss in the combustion reaction is equal to the heat gain by water. Therefore, the quantity of heat changed will be:
Therefore, Q = 11760 J
Q.2: If Sodium chloride is dissolved in 100g of water at 20^ C, the after proper stirring temperature will be \. Compute the heat change during the process of dissolution, if the specific heat capacity of the solution is \.
Solution: Given parameters are,
- \
- \
- \
Since heat absorbed by the salt will be the same as Heat lost by water. Therefore We have the formula,
Therefore, Q = 1672 J
Enthalpy Of Phase Transitions
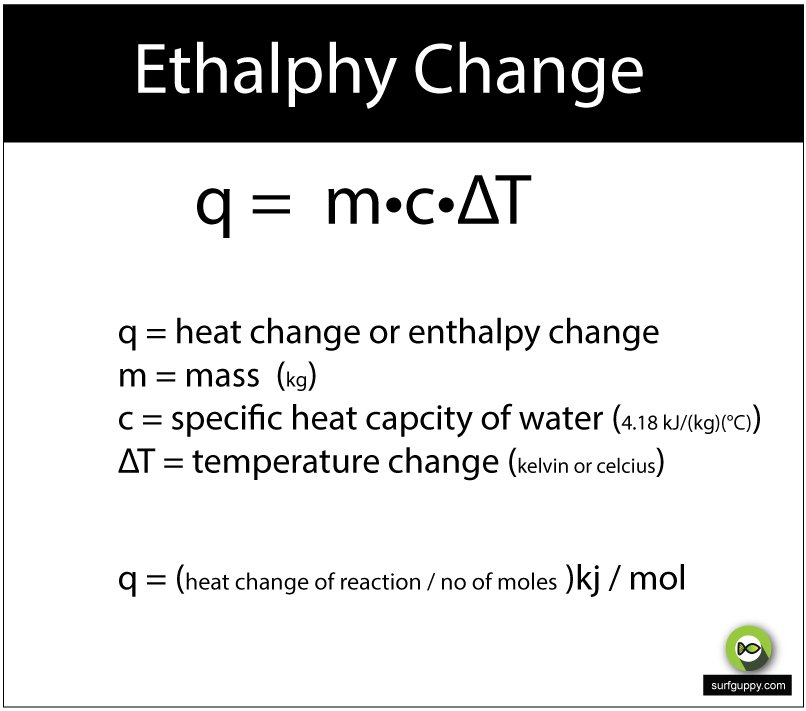
When a substance changes from solid to liquid, liquid to gas or solid to gas, there are specific enthalpies involved in these changes. The enthalpy of melting describes the transition from solid to liquid , the enthalpy of vaporization describes the transition from liquid to gas and the enthalpy of sublimation describes the transition from solid to gas .
For water, the enthalpy of melting is Hmelting = 6.007 kJ/mol. Imagine that you heat ice from 250 Kelvin until it melts, and then heat the water to 300 K. The enthalpy change for the heating parts is just the heat required, so you can find it using:
H = nCT
Where is the number of moles, is the change in temperatue and is the specific heat. The specific heat of ice is 38.1 J/K mol and the specific heat of water is 75.4 J/K mol. So the calculation takes place in a few parts. First, the ice has to be heated from 250 K to 273 K . For 5 moles of ice, this is:
H = nCT
Sum these parts to find the total change in enthalpy for the reaction:
Htotal = 10.179 kJ + 30.035 kJ + 4.382 kJ
= 44.596 kJ
Don’t Miss: Draw A Lewis Structure (including All Lone Pair Electrons) For The Species Ccl4:
Introduction To The Heat Of Combustion
Combustion reactions are known as one of the most important and most frequent chemical reactions taking place in our surroundings. The reason why these reactions are so important lies in the fact that a tremendous amount of energy is released as a byproduct.
We used this heat of combustion as fuel to run automobiles and other gas equipment. Yes, the fuel over which you run your cars and other vehicles burn in the combustion chemical reaction and we guess, this point is enough to predict the importance of combustion.
Most combustion reactions occur when hydrocarbons react with oxygen to form carbon monoxide and water. Since we study all the hydrocarbons in organic chemistry, the heat evolved by their combustion chemical reaction is called heat of combustion of organic compounds.
To understand it further, let’s have a look at the combustion reaction examples, Let’s start from methanol
In the given reaction, we can see that one mole of methanol is reacting with one mole of oxygen to produce one mole of CO2 and two moles of water. Since the heat of combustion is released only by the one mole of reacting substances, we will call it the molar heat of combustion.
Calculating Heat Of Combustion
The tool we usually use to find the heat of combustion of any organic compound is called a bomb calorimeter. For this purpose, all you have to do is to burn your sampling compound in the calorimeter and wait.
However, while using a calorimeter, it is important to keep the oxygen concentration higher. Otherwise, the process of incomplete combustion gets started.
Similarly, you can also calculate the heat of combustion by observing the temperature change in the system. Lets calculating it using combustion reaction examples.
Also learn step by step equation balancing procedure and how to write chemical equation for an endothermic reaction.
You May Like: Eoc Fsa Warm Ups Algebra 1 Answers
Using A Material’s Specific Heat
Theory Behind Determining Molar Enthalpy Of Solution
The molecules or ions making up a solid solute exist in a highly ordered state which is referred to as a lattice.You are probably already familiar with representations of ionic compounds in which positive ions and negative ions are arranged in a lattice held together by electrostatic forces of attraction known as ionic bonds.The amount of disorder, or randomness, in a system is known as its entropy.A lattice is highly ordered, that is, the particles making up the lattice are in a low state of disorder.This is referred to as a low entropy state3.
When this solute dissolves in a solvent, particles such as ions must be removed from the lattice and each solute particle must then be completely surrounded by solvent molecules.The solute particles in the solution are in constant motion and distributed more or less randomly throughout the solution so that the amount of disorder has increased compared to when they were part of the lattice.Solute particles in the solution are said to be in a higher state of entropy than solute particles making up the lattice.
The process of dissolving a solid solute in a liquid solvent can therefore be thought of as occurring in two steps:
| Step 1: A particle of solute, such as an ion or molecule, breaks away from the lattice. |
Play the game now!
Read Also: Who Are Paris Jackson’s Biological Parents
Calculation Based On Heat Of Formation
1. When 5g of liquid water was formed by burning hydrogen gas, +65KJ of heat was given off. Calculate the standard heat of formation of water.
H2 + ½O2 H2O Hf = ?KJ mol-1
H2O = 18
Solution:
If 5g of water produces +65KJ of heat, 1g of liquid water produces +65/5 KJ of heat.
18g of liquid water produces:
2. When 4.5g of liquid water was formed by burning hydrogen gas in oxygen -72KJ of heat was given off. Calculate the standard heat formation of water.
H2 + ½ O2 H2O Hf = ?
Solution:
If 4.5g of liquid water produces -72KJ of heat, 1g of liquid water produces :- \
Therefore 18g of liquid water will produce:
Experiment To Determine The Molar Heat Of Solution Of A Solute
The following describes the use of a polystyrene foam cup as a calorimeter to determine the heat of solution of a salt in water .5
Polystyrene foam is a good insulator, that is, it is a material that does not conduct heat well.
In this experiment the polystyrene foam cup is used as an insulated vessel in which to conduct the experiment so that either:
all the energy released by the reaction is used to raise the temperature of the water in the cup and is not lost in heating the surrounding environment in the case of an exothermic reaction
the only energy being absorbed by the reaction is that from the water in the cup and not from the surrounding environment in the case of an endothermic reaction.
Procedure:
Don’t Miss: What Does Abiotic Mean In Biology
Key Takeaways: Heat Of Fusion For Melting Ice
- Heat of fusion is the amount of energy in the form of heat needed to change the state of matter from a solid to a liquid
- The formula to calculate heat of fusion is: q = m·Hf
- Note that the temperature does not actually change when matter changes state, so it’s not in the equation or needed for the calculation.
- Except for melting helium, heat of fusion is always a positive value.
Heat Of Formation \ \
The standard enthalpy of formation is the heat evolved or absorbed when one mole of substance is formed from its elements under standard conditions. The standard heat of formation could be endothermic or exothermic i.e positive or negative. Example
i. Standard enthalpy of formation of water
H2 + ½ O2 H2O Hf = -285KJ mol-1
ii. Standard enthalpy of formation of hydrogen iodide
½ H2 + ½ I2 HI Hf = +26.0KJ mol-1
iii. Standard enthalpy of formation of carbonoxide
C + O2 CO2 Hf = -393KJ mol-1
Thus, the more exothermic the standard heat of formation of a compound, the greater is its energetic stability. Hence CO2 is more stable with respect to its elements than H2O, because more heat is liberated during the formation of CO2 than water. Enthalpy of formation is zero for element in their normal state, e.g carbon, hydrogen, sulphur, iodine, etc.
Recommended Reading: Elastic Force Examples
How To Calculate Specific Heat
If you have problems with the units, feel free to use our temperature conversion or weight conversion calculators.
How To Calculate Specific Heat Capacity
Also Check: Dot Structure For Ccl4
How To Calculate Enthalpy Change
The enthalpy change of a reaction is the amount of heat absorbed or released as the reaction takes place, if it happens at a constant pressure. You complete the calculation in different ways depending on the specific situation and what information you have available. For many calculations, Hesss law is the key piece of information you need to use, but if you know the enthalpy of the products and the reactants, the calculation is much simpler.
TL DR
You can calculate changes in enthalpy using the simple formula: H = Hproducts Hreactants
Typical Values Of Specific Heat
You don’t need to use the heat capacity calculator for most common substances. The values of specific heat for some of the most popular ones are listed below.
- ice: 2,100 J/
- water vapor: 2,000 J/
- basalt: 840 J/
- copper: 380 J/
- lead: 130 J/
Having this information, you can also calculate how much energy you need to supply to a sample to increase or decrease its temperature. For instance, you can check how much heat you need to bring a pot of water to the boil to cook some pasta.
Wondering what the result actually means? Try our potential energy calculator to check how high you would raise the sample with this amount of energy. Or check how fast could the sample move with this kinetic energy calculator.
Don’t Miss: Theory Of Everything Geometry Dash 2