What Is The Distillation Process
- What is the Distillation Process?
Distillation is the process of purifying a substance, whereby pure substances are extracted from a mixture.
There are different types of distillation processes, including fractional distillation, simple, steam, and vacuum distillation.
Distillation has several commercial and industrial applications. For example, it can be used to distil wine. In fact, the well-documented use of distillation can be traced as far back as the 13th century, where it was used to distil alcohol from wine. Itâs able to do this because the difference in the boiling point of water and ethanol makes it possible to isolate purer amounts of alcohol from wine.
Another major application of distillation is in the petroleum industry, where fractional distillation is used to purify crude oil into different types of commercial fuels, like gasoline and diesel.
In this post:
The distillation process generally involves three main steps:
Specific types of distillation processes may have several more stages, such as the fractional distillation of crude oil .
In general, heat, corresponding to the boiling point of the desired liquid, is applied to the mixture that will be distilled. In other instances, pressure is lowered to optimise the distillation process.
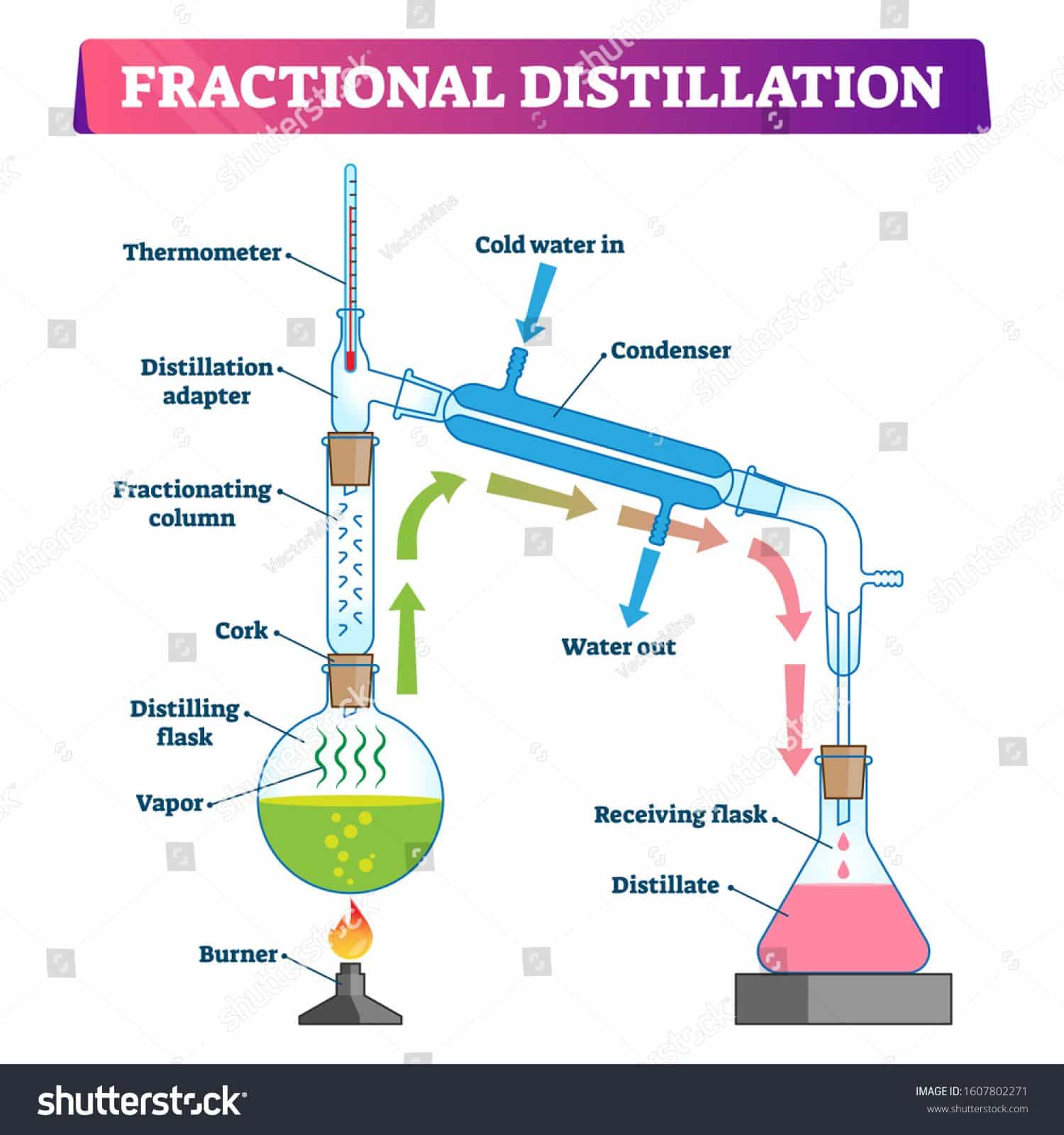
Below is an illustration of a basic laboratory distillation setup:
What Is An Example Of Distillation In Chemistry
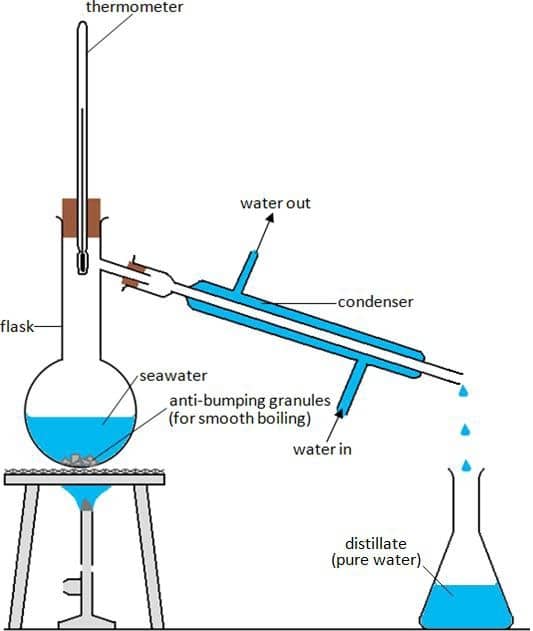
Distillation is widely used in industry. For example, distillation can be used to purify water, especially in those areas where freshwater is inaccessible. So, they use distillation to purify seawater and make it safe for drinking. Water distillation is also used to remove minerals and other impurities.
Raoults Law And Daltons Law
The distillation process is dependent on the two laws, and they are Daltons Law and Raoults Law for a mixture of liquids. According to Daltons law of partial pressures, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of all the constituent gases. Raoults law, on the other hand, states that the partial pressure of a single liquid component in an ideal liquid mixture equals the product of the vapor pressure of the pure component and its mole fraction.
It is to be noted that the boiling point of the liquid depends on the surrounding pressure and changes accordingly. For instance, the boiling point of water at sea level is 100 degrees Celcius. However, its boiling point at an altitude of 1905 meters is 93.4 degrees Celsius as the pressure of the atmosphere is relatively lower at high altitudes.
A plant performing the process of distillation is called a distillery. When the distillation is performed in a laboratory, it uses batches of the liquid mixture, whereas, in industrial distillation processes, which are generally continuous, a constant composition of the mixture requires to be maintained.
Don’t Miss: What Is An Example Of Movement In Geography
Distillation For Compound Purification
Simple distillations are used frequently in the organic chemistry teaching labs. They are useful in the following circumstances:
- the liquid is relatively pure to begin with
- the liquid has a non-volatile component, for example, a solid contaminant
- the liquid is contaminated by a liquid with a boiling point that differs by at least 70°C
“Simple” distillation may be a misleading term to the beginning organic chemistry student, since it takes a lot of practice in simple distillation to become proficient in this technique. It is especially important to do a perfect simple distillation when determining a boiling point for identification purposes. You can see detailed photos of a simple distillation set-up here. Be sure to have correct placement of the thermometer, fill the flask to the correct level, and use a boiling chip.
Breaking An Azeotrope With Unidirectional Pressure Manipulation
The boiling points of components in an azeotrope overlap to form a band. By exposing an azeotrope to a vacuum or positive pressure, it’s possible to bias the boiling point of one component away from the other by exploiting the differing vapor pressure curves of each the curves may overlap at the azeotropic point, but are unlikely to remain identical further along the pressure axis to either side of the azeotropic point. When the bias is great enough, the two boiling points no longer overlap and so the azeotropic band disappears.
This method can remove the need to add other chemicals to a distillation, but it has two potential drawbacks.
Under negative pressure, power for a vacuum source is needed and the reduced boiling points of the distillates requires that the condenser be run cooler to prevent distillate vapors being lost to the vacuum source. Increased cooling demands will often require additional energy and possibly new equipment or a change of coolant.
Alternatively, if positive pressures are required, standard glassware can not be used, energy must be used for pressurization and there is a higher chance of side reactions occurring in the distillation, such as decomposition, due to the higher temperatures required to effect boiling.
A unidirectional distillation will rely on a pressure change in one direction, either positive or negative.
Don’t Miss: What Is Single Replacement In Chemistry
Distillation In Food Processing
- Main article: Distilled beverage
Carbohydrate-containing plant materials are allowed to ferment, producing a dilute solution of ethanol in the process. Spirits such as whiskey and rum are prepared by distilling these dilute solutions of ethanol. Other components than ethanol are collected in the condensate, including water, esters, and other alcohols which account for the flavor of the beverage.
Distillation Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
In the most general sense, “distillation” means to purify something. For example, one you might distill the main point from a story. In chemistry, distillation refers to a particular method of purifying liquids:
Read Also: How To Teach Sat Math
Is Distillation A Chemical Or Physical Process
Distillation is a physical process because it involves a phase change from liquid to gas , and then back again to liquid. Generally, no chemical change is intended to occur during the process of distillation. That said, some incidental or accidental chemical reactions may occur during the process of distillation. The risk of this happening increases as the scale becomes larger.
For example, the distillation of flammable liquids may result in combustion or even explosion. In some cases, liquids may also react with the components of the distillation equipment. Some liquids may even react with the oxygen or impurities in the air once vaporised. Watch this case study presentation about an accidental explosion at a vinyl chloride monomer distillation plant.
What Is Distillation Process In Chemistry
Distillation is a process where a liquid mixture is separated into its components by continuous vaporization and then condensation.
Suppose we have a mixture of two different liquids: liquid A and liquid B . When the mixture is heated, liquid A will vaporize faster. At this temperature, the vapor will consist of a large percentage of compound A, leaving the rest of the liquid enriched in compound B. The liquid A vapor will pass through the condenser, which will turn it back into a liquid. Then, liquid A will be collected in another flask.
After we collect liquid A, we can keep heating the mixture , until it reached its boiling point, and we can collect it in another flask.
Also Check: Algebra 2 Mid Year Test Study Guide Answer Key
Distillation For Boiling Point Determination
The organic teaching labs employ distillation routinely, both for the identification and the purification of organic compounds. The boiling point of a compound, determined by distillation, is well-defined and thus is one of the physical properties of a compound by which it can be identified. Distillation is used to purify a compound by separating it from a non-volatile or less-volatile material. Because different compounds often have different boiling points, the components often separate from a mixture when the mixture is distilled.
The boiling point is the temperature at which the vapor pressure of the liquid phase of a compound equals the external pressure acting on the surface of the liquid. The external pressure is usually the atmospheric pressure. For instance, consider a liquid heated in an open flask. The vapor pressure of the liquid will increase as the temperature of the liquid increases, and when the vapor pressure equals the atmospheric pressure, the liquid will boil. Different compounds boil at different temperatures because each has a different, characteristic vapor pressure: compounds with higher vapor pressures will boil at lower temperatures.
If you are using the boiling point to identify a solid compound which you have isolated in the lab, you will need to compare its boiling point with that of the true compound. Boiling points are listed in various sources of scientific data, as referenced on the Chemical Information page on this website.
Distillation Examples In Chemistry
Distillation is widely used in industry. For example, distillation can be used to purify water, especially in those areas where freshwater is inaccessible. So, they use distillation to purify seawater and make it safe for drinking. Water distillation is also used to remove minerals and other impurities.
Another application of distillation is the recycling of oils. By using distillation, oil can be purified by removing water and other contaminants.
You May Like: 3.1 Puzzle Time Answer Key Algebra 2
Distillate Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
A distillate is the vapor in a distillation that is collected and condensed into a liquid. Alternatively, it is the name of the product obtained from the distillation process.
Applications Of Steam Distillation
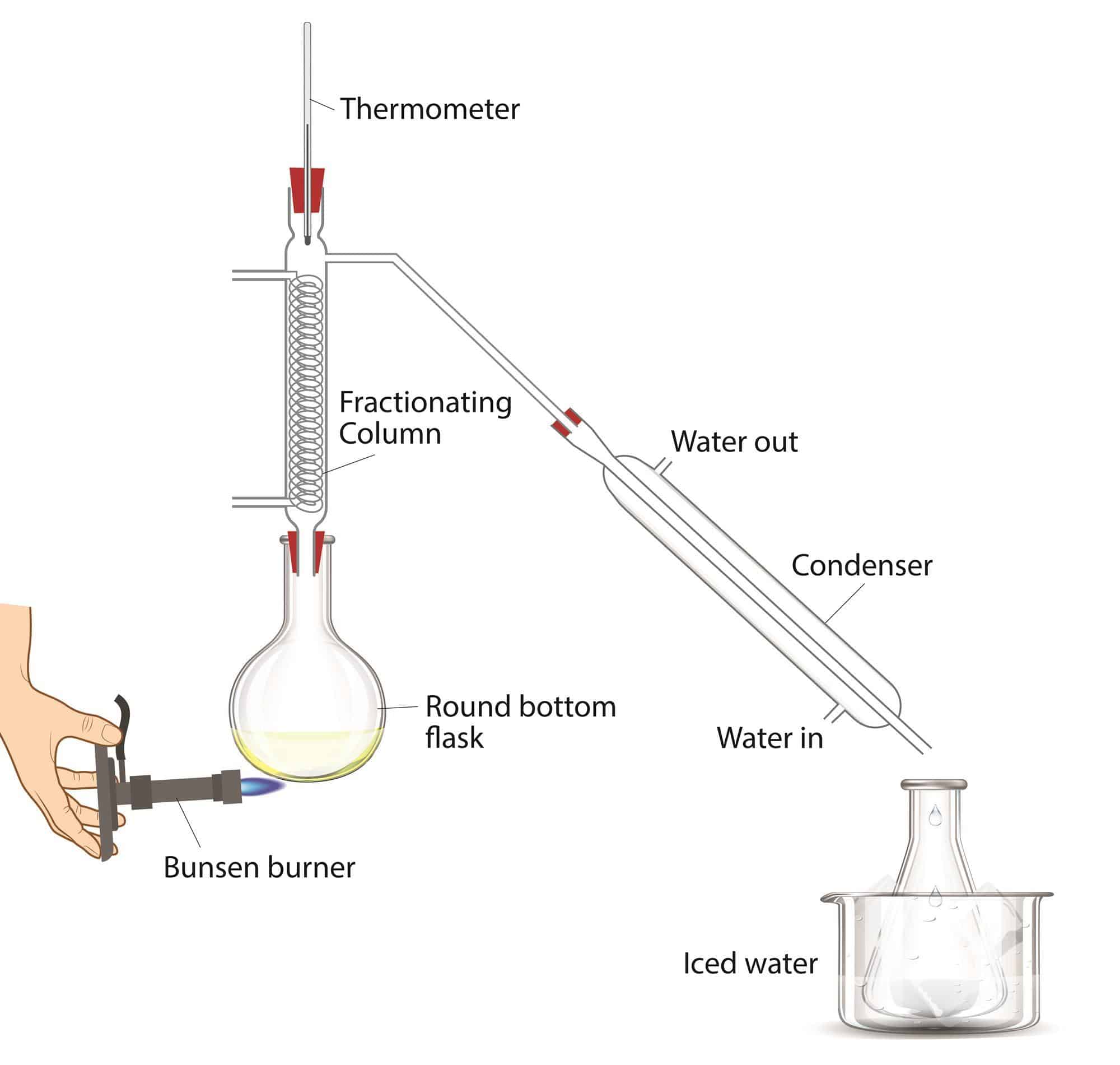
Steam distillation are widely used in the manufacturing of essential oils, for instance perfumes. This method uses a plant material that consists of essential oils. Mainly orange oil is extracted on a large scale in industries using this method.
The application of steam distillation can be found in the production of consumer food products and petroleum industries. They are used in the separation of fatty acids from mixtures.
Recommended Reading: Who Is The Father Of Chemistry Biology And Physics
Various Examples Of Distillation
1. The simplest and most common example of distillation is the steam from a kettle, which gets deposited as drops of distilled water on a cold surface.
2. This process is used in the separation of alcoholic liquors from fermented materials for purification of alcohol by separating liquids from non-volatile solids.
3. It is used to separate two or more liquids having different boiling points, as in the case of separation of gasoline, kerosene, and lubricating oil from crude oil in crude oil refining, enabling safe storage and transportation.
4. Making liquefied gases from the air. For example, nitrogen, oxygen, and argon are distilled from the air.
5. The desalination of seawater is done through distillation.
6. Perfumes and flavorings for food are obtained from herbs and plants by distillation.
7. Distilled water is used in lead-acid batteries and low-volume humidifiers.
8. Other industrial uses include the processing of chemical products such as formaldehyde and phenol.
References:
Simple Distillation Vs Fractional Distillation
These two processes are highly similar in nature, as they have the same purpose however, one can be chosen over the other based on boiling point similarities. As used in the example above, sometimes a mixture of liquids will have very different boiling points and it can be simple to control this procedure to yield close to pure results. Here you would use simple distillation. However, sometimes a mixture will contain liquids with boiling points that can differ by only a few degrees when this chemical property is similar, it can be hard to separate the liquids in one run. Fractional distillation, also known as differential distillation, helps with this problem through the use of extra equipment.
Read Also: What Is Spur In Geography
Role Of Raoults Law And Daltons Law
The temperature at which the vapor pressure of a liquid becomes equal to the pressure of the surrounding area is known as the boiling point of that liquid. At this temperature point, the liquid is converted into its vapor form via the formation of vapor bubbles at its bulk.
It is important to note that the boiling point of the liquid changes with the surrounding pressure. For example, the boiling point of water at sea level is 100oC but its boiling point at an altitude of 1905 meters is 93.4oC .
For a mixture of liquids, the distillation process is dependent on Daltons law and Raoults law. As per Raoults law, the partial pressure of a single liquid component in an ideal liquid mixture equals the product of the vapor pressure of the pure component and its mole fraction. According to Daltons law of partial pressures, the total pressure exerted by a mixture of gases is equal to the sum of the partial pressures of all the constituent gases.
When a mixture of liquids is heated, the vapor pressure of the individual components increases, which in turn increases the total vapor pressure. Therefore, the mixture cannot have multiple boiling points at a given composition and pressure.
Short Path And Molecular Distillation
Molecular distillation is vacuum distillation below the pressure of 0.01 torr. 0.01 torr is one order of magnitude above high vacuum, where fluids are in the free molecular flow regime, i.e. the mean free path of molecules is comparable to the size of the equipment. The gaseous phase no longer exerts significant pressure on the substance to be evaporated, and consequently, rate of evaporation no longer depends on pressure. That is, because the continuum assumptions of fluid dynamics no longer apply, mass transport is governed by molecular dynamics rather than fluid dynamics. Thus, a short path between the hot surface and the cold surface is necessary, typically by suspending a hot plate covered with a film of feed next to a cold plate with a line of sight in between. Molecular distillation is used industrially for purification of oils.
Read Also: What Is An Inhibitor Chemistry
Main Difference Reflux Vs Distillation
Reflux and distillation are two chemical techniques. Reflux is a technique that involves the condensation of vapors which are then returned back to the sample. It is used in laboratory distillation processes. Distillation is the action of purifying a liquid by a process of heating and cooling. Distillation can be found in four major types as simple distillation,fractional distillation, steam distillation and vacuum distillation. The main difference between reflux and distillation is that reflux method is used to complete a certain chemical reaction whereas distillation is used to separate components in a mixture.
Why Is It Impossible To Completely Purify A Mixture By Distillation
At the boiling point of a mixture of liquids, all the volatile constituents boil. However, the quantity of a constituent in the resulting vapor is based on its contribution to the total vapor pressure of the mixture. This is why the compounds with higher partial pressures can be concentrated in the vapors whereas the compounds having low partial pressures can be concentrated in the liquid.
Since a component in the mixture cannot have zero partial pressure, it is impossible to obtain a completely pure sample of a component from a mixture via distillation. However, samples of high purity can be obtained when one of the components in the mixture has a partial pressure which is close to zero.
You May Like: How Do Airbags Work Physics
Distillation Of Crude Oil
Crude oil is a mixture of many hundreds of liquid hydrocarbons. Dissolved in it are many other hydrocarbons some of which are solids and some gases . There may also be some hydrocarbon gases trapped above the oil, as for example in some of the oil fields in the North Sea.
In the refineries the oil is distilled into liquid fractions with different boiling point ranges which are then further processed.
The crude oil is heated in a furnace and the resulting mixture fed as a vapour into a fractionating tower which can have a height of 25-100 m, handling volumes of over 40 000 m3 a day. The column may contain 40-50 steel sieve trays which fit horizontally across the column and are designed to ensure there is intimate mixing between the descending liquid, formed by condensation, and the rising vapour. To effect this close contact, the trays have holes in them through which the vapour flows up into the liquid collecting on the trays .
Figure 2 Trays in a fractionating column.
Alternatively, there may be valves fitting over the holes, which lift up when the pressure of the vapour below the tray is greater than the pressure on the tray . These are considered to be more efficient in fractionation than sieve trays without valves.
Figure 3 Valve trays.
Figure 4 This FLEXITRAYTM valve tray is a steel sheet on which liftable valves are mounted.They are much more efficient than sieve trays.By kind permission of Koch-Glitsch, LP.
Figure 9 The fractional distillation of crude oil.
Batch Or Differential Distillation
Heating an ideal mixture of two volatile substances, A and B, with A having the higher volatility, or lower boiling point, in a batch distillation setup until the mixture is boiling results in a vapor above the liquid that contains a mixture of A and B. The ratio between A and B in the vapor will be different from the ratio in the liquid. The ratio in the liquid will be determined by how the original mixture was prepared, while the ratio in the vapor will be enriched in the more volatile compound, A . The vapor goes through the condenser and is removed from the system. This, in turn, means that the ratio of compounds in the remaining liquid is now different from the initial ratio .
The result is that the ratio in the liquid mixture is changing, becoming richer in component B. This causes the boiling point of the mixture to rise, which results in a rise in the temperature in the vapor, which results in a changing ratio of A : B in the gas phase . This results in a slowly changing ratio of A : B in the distillate.
If the difference in vapour pressure between the two components A and B is large generally expressed as the difference in boiling points the mixture in the beginning of the distillation is highly enriched in component A, and when component A has distilled off, the boiling liquid is enriched in component B.
Recommended Reading: What Does Msw Stand For In Psychology