How Can I Prevent Corrosion
One of the best ways to prevent corrosion is to apply an Anti-Corrosion Protective Coating. A protective coating protects its substrate by preventing contact between the substrate and harsh environments . Here at CSL Silicones Inc, we offer two kinds of anti-corrosion protective coatings that are easily applied using only one coat. The Si-COAT® 579 AC protective coating is cost-effective and offers long-lasting protection to virtually any substrate.
The coatings are environmentally responsible, have superior temperature resistance , will not chalk or fade, have a low film build, require only a single-coat of application, and have outstanding UV resistance. The 180% elasticity makes the coating highly flexible, which allows for thermal expansion and contraction of the substrate to which it is applied.
The Advantages Of Coatings
There are numerous benefits to using coatings to prevent corrosion. Here are some of the most common reasons:
- Coatings can be applied onsite, as opposed to galvanizing.
- Coatings can stabilize the surface of steel so it wont react to the corrosive factors in the environment.
- Coatings can cover the substrate, leaving no gaps for corrosion to begin forming.
Other Methods Of Protection
In some cases, it helps to add compounds to the metal which increase its durability to corrosion . Other metals are also used as additives which are less prone to rusting, or non-organic or organic compounds.
Inhibitors of corrosion are substances that prevent and stop metal damage. Usually, adding them changes the properties of the environment the metal is in, making it favorable for keeping the surface intact. Various substances may be used as inhibitors depending on the type of metal and cause of corrosion. For example, to prevent iron rusting, potassium bichromate or amines may be introduced into the environment, if it is damp. Click here for amazing experiments with iron.
In some cases, inhibitors are not introduced, but more aggressive elements that cause corrosion are removed from the environment .When using home methods of rust removal, it is important to observe caution, as this often involves working with caustic substances. All mechanical abrasives are quite rough, so one should work in protective gloves to avoid scratches.
Recommended Reading: Segment Addition Postulate Color By Number Worksheet Answer Key
Statue Of Liberty: Changing Colors
The Statue of Liberty is a landmark every American recognizes. The Statue of Liberty is easily identified by its height, stance, and unique blue-green color . When this statue was first delivered from France, its appearance was not green. It was brown, the color of its copper skin. So how did the Statue of Liberty change colors? The change in appearance was a direct result of corrosion. The copper that is the primary component of the statue slowly underwent oxidation from the air. The oxidation-reduction reactions of copper metal in the environment occur in several steps. Copper metal is oxidized to copper oxide , which is red, and then to copper oxide, which is black
Coal, which was often high in sulfur, was burned extensively in the early part of the last century. As a result, sulfur trioxide, carbon dioxide, and water all reacted with the CuO
These three compounds are responsible for the characteristic blue-green patina seen today. Fortunately, formation of the patina created a protective layer on the surface, preventing further corrosion of the copper skin. The formation of the protective layer is a form of passivation, which is discussed further in a later chapter.
Figure 1.
Perhaps the most familiar example of corrosion is the formation of rust on iron. Iron will rust when it is exposed to oxygen and water. The main steps in the rusting of iron appear to involve the following . Once exposed to the atmosphere, iron rapidly oxidizes.
Figure 2.Figure 3.
What Is A Corrosion Inhibitor
A chemical compound that can be added to liquids or gases and used to decrease the corrosion rate of a given material can be referred to as a corrosion inhibitor.
One method for the inhibition of corrosion would be the addition of a coating on the surface of the metal which acts as a passivation layer and disallows access to the surface of the metal.
Also Check: Geometry Basics Segment Addition Postulate Worksheet Answer Key
Precise Measurements Are The Molecular Lens To Seeing Chemistry
The analysis techniques the team uses are surface-sensitive techniques: polarized modulated-infrared reflection-absorption spectroscopy , attenuated total reflectance-Fourier transform infrared spectroscopy, X-ray photoelectron spectroscopy and atomic force microscopy .
The spectroscopy tells us the chemistry the microscopy tells us the physical changes, Perrine said. Its really difficult to image these corrosion experiments in real-time with AFM because the surface is constantly changing, and the solution is changing during corrosion.
What the images do reveal is a sequence of pitting, chewing and degrading the surface, known as corrosion, which produces nucleation sites for the growth of minerals. The key part is watching the initial stages as a function of time.
We can watch the corrosion and film growth as a function of time. The calcium chloride tends to corrode the surface faster, because we have more chloride ions, but also has a faster rate of carbonate formation, Perrine said its possible to see how sodium chloride solution corrodes the surface of iron gradually and continues forming rust as the solution dries.
She adds that since iron is ubiquitous in environmental systems, slowing down and closely observing mineral formation comes down to adjusting the variables in how it transforms in different solutions and exposure to air.
Corrosion And Corrosion Prevention
Iron is a useful metal, but it rusts in the atmosphere. Rusting can be prevented by keeping oxygen and water away, and by sacrificial protection.
can oxidise in air. They react with oxygen and form metal oxides. For example, sodium is a very reactive metal. When sodium is cut or scratched, its freshly exposed shiny surface rapidly turns dull as a thin layer of sodium oxide forms:
sodium + oxygen sodium oxide
4Na + O2 2Na2O
Other metals may oxidise more slowly. Gold and other very unreactive metals do not oxidise in air at all.
happens when a metal continues to oxidise. The metal becomes weaker over time, and eventually all of it may become metal oxide.
Don’t Miss: What Does Abiotic Mean In Biology
Corrosion Of Noncrystalline Materials
The aqueous corrosion in glasses was classified into two types: Static and dynamic. In static aqueous corrosion, there is an entrapment of moisture on the surface of the glass. In dynamic aqueous corrosion, the corrosion solution is replenished due to condensation run-off.
-
Corrosion by solid
The corrosion behavior of noncrystalline materials in contact with solid was similar to the crystalline ceramic, which causes degradation of the materials through chemical interdiffusion processes.
-
Corrosion by liquid
The corrosion of glasses by liquid follows chemical dissolution, which was similar to that of corrosion in crystalline ceramics . The aqueous media of acids and alkalis and even neutral liquid media also corrode the surface of glasses by dissolution process.
-
Corrosion by gas
Ceramic materials undergo rapid corrosion by vapor attack and the severity in the degradation was due to the availability of larger surface area for the vapors to contact than the liquids or solids . In noncrystalline glasses, the contact of gases to the glass surface results in dissolution of the surface, thereby causing corrosion.
In most cases during gaseous corrosion, the oxidation and dissolution processes are more prevalent form of attack and in some cases hydrogen reactions are also possible .
Ajit Behera, … Deepak Kumar Sahoo, in, 2020
Vapor Phase Inhibitors/volatile Corrosion Inhibitors
Vapor phase corrosion inhibitors are volatile compounds introduced in a closed system for corrosion protection. These inhibitors possess a high vapor pressure at normal temperature and protect the metal by forming a bond and barrier layer on the metal surface. In general, a weak volatile acid or base that easily hydrolyzes provides the most effective inhibition. Volatile alkaline compounds such as octadecylamine and morphine are introduced in boilers with steam to inhibit corrosion in condenser tubes by neutralizing the acidic carbon dioxide. Inhibiting anions such as nitrite and amine substituted into organic structures increase vapor pressure, increasing their protective ability against aggressive ions in atmospheric corrosion . The inhibition mechanism of VCI is not clear. It is assumed that VCI forms a weak bond as an adsorbed monolayer that protects the surface from water inclusion and aggressive ions such as sulfates and chlorides. The adsorbed monolayer changes anodic and cathodic reaction kinetics . Vapor phase inhibitors used in corrosion protection are summarized in Table 14.1.
Table 14.1. Vapor Pressure Inhibitor
| Substance |
|---|
L.M. Calle, W. Li, in, 2014
Don’t Miss: Who Are Paris Jackson’s Parents
How The Rusting Process Takes Place
Metal corrosion can be observed quite frequently in open air in a damp environment insoluble compounds form on the surface, oxides , carbonates , and sulfides of the corresponding metal.A typical example of rusting is the appearance of reddish-brown coatings on steel objects . The equation of the process is the following:
4Fe + 6HO + 3O = 4Fe hydroxide an insoluble base of a reddish-brown color)
When this reaction takes place, access of air and moisture making contact with the metal surface are sufficient. As Fe in this form is quite an unstable compound, it swiftly loses water, forming the oxide FeO:
2Fe = FeO + 3HO
Iron oxide is not an oxide film which can protect the metal from further destruction. The formation does not stop subsequent oxidation of the metal on the surface of FeO, so if the rust is not removed in time, the metal may be destroyed completely.
This does not happen with all metals in the corrosion process, some are covered with a thick oxide film, which protects the metal surface from further destruction. Only after its removal, the metal begins to react with the environment again
4Al + 2HO + 3O = 4AlO
Low reactive metals corrode much more slowly and weakly than active ones. Noble metals are barely damaged at all under the impact of the environment gold Au, silver Ag, platinum Pt etc.
Uses Of Corrosion Inhibitors
Corrosion Inhibitors have a wide range of uses in commercial, process, and industrial environments. Some of these uses are listed below.
- Corrosion Inhibitors are used to stop rusting and anodic corrosion of metals. This is generally done via the coating of the metal surface with a chromate layer.
- Oxygen scavengers can be used as CIs to react with dissolved oxygen in the environment and can help in preventing cathodic corrosion.
- It is very important to prevent rusting and corrosion of fuel pipelines. Therefore, CIs are very important in securing these pipelines and reducing the risk of accidents.
- Metal pipes in heating systems are prone to corrosion. CIs play an important role in securing these pipes as well.
Don’t Miss: Who Are Paris Jackson’s Biological Parents
Removing And Treating Rust
Depending on the situation and application, you may be able to treat the area that has corroded. If the affected area is small and treatable, you may require some tools and products to remove it. Begin by removing the rust from the metal using a tools such as a grinding wheel or needle gun. Be careful not to cause any additional damage to the metal.
For large corroded areas, you may want to consider a permanent protective coating, such as CSLs SI-COAT Anti Corrosion Protective Coating. You will also want to take this time to look at the application as a whole for other premature signs of corrosion.
New Surface Chemistry Technique Reveals Corrosions Secrets

One can easily see with the naked eye that leaving an old nail out in the rain causes rust. What does require the keen eyes and sensitive nose of microscopy and spectroscopy is observing how iron corrodes and forms new minerals, especially in water with a pinch of sodium and calcium.
Thanks to a new technique developed by chemists at Michigan Technological University, the initial stages of this process can be studied in greater detail with surface analysis. The team, led by Kathryn Perrine, assistant professor of chemistry, recently published their latest paper in The Journal of Physical Chemistry A.
The groups main finding is that the cation in solution positively charged sodium or calcium ions influences the type of carbonate films grown when exposed to air, which is composed of atmospheric oxygen and carbon dioxide. The gradual exposure of oxygen and carbon dioxide produces carbonate films specific to the cation. The iron hydroxides of different shapes and morphologies are without gradual air exposure, not specific to the cation.
A better understanding of this process and how fast the minerals form opens up possibilities for monitoring carbon dioxide capture, water quality byproducts and improving infrastructure management for old bridges and pipes.
Don’t Miss: Paris Jackson Biological Father Mark Lester
Changes In Environmental Conditions
Finally, the corrosion rate can be stopped or reduced with the alteration of the environmental conditions in which the metallic material is found.
The humidity and contents of sulfur, chlorides and oxygen in liquids and gases must be kept at low levels to increase the life expectancy of a material, and using less saline and / or hard water has a positive effect.
How To Remove Corrosion At Home
Metallic, non-metallic coatings and other methods of protection
Corrosion is the spontaneous destruction of metal under the impact of the environment. Any factors can have a negative impact on metal chemical, physico-chemical, electrochemical. Not only pure metals are subject to corrosion many alloys may also rust.
Recommended Reading: Age World Problems
How To Remove Rust At Home
Traces of rust often appear on the surface of iron objects. If destruction has begun recently, and the surface of the metal is not damaged too greatly, then rust can be removed at home.
Often the simplest method is used to do this the mechanical removal of rust by a rough metal brush. To achieve the effect more quickly, you can prepare a paste of baking soda or hydrogen peroxide with cream of tartar, rub the surface of the metal with the paste, and then rub it off.For large objects, rough sanding equipment and machines are used. They work according to the same principle as rough brushes when the disk rotates, the rust is removed. It is important to remove the corrosion carefully, as it is possible that the machine may damage the clean surface of the metal.
Any acids are especially good for removing rust by reacting with iron oxides and hydroxides, they dissolve unwanted formations.
Generally, there are a few simple methods for removing rust with acids:
- soaking in apple vinegar
- soaking in lemon or lime juice
- soaking in hydrochloric or phosphoric acid . The orthophosphoric acid contained in Coca Cola means it can also be used to remove rust from metal.
Rust can be removed from small objects by placing a potato on a metallic surface or sticking an object into a potato the oxalic acid contained in potatoes also provides effective protection against corrosion.
Corrosion Examples Reactions And Effects
Here are some of the typical examples of corrosion as seen mostly in metals.
1. Copper Corrosion
When copper metal is exposed to the environment it reacts with the oxygen in the atmosphere to form copper oxide which is red in colour.
2Cu + ½ O2 Cu2O
Cu2O further gets oxidised to form CuO which is black in colour.
Cu2O + ½ O2 2CuO
This CuO reacts with CO2, SO3 and H2O 2 which is blue in colour and Cu4SO46 which is green in colour.
This is why we observe copper turning bluish-green in colour.
A typical example of this is the colour of the statue of liberty which has the copper coating on it turning blue-green in colour.
2. Silver Tarnishing
Silver reacts with sulphur and sulphur compounds in the air give silver sulphide which is black in colour. Exposed silver forms Ag2S as it reacts with the H2S in the atmosphere which is present due to certain industrial processes.
2Ag + H2S Ag2S + H+2+
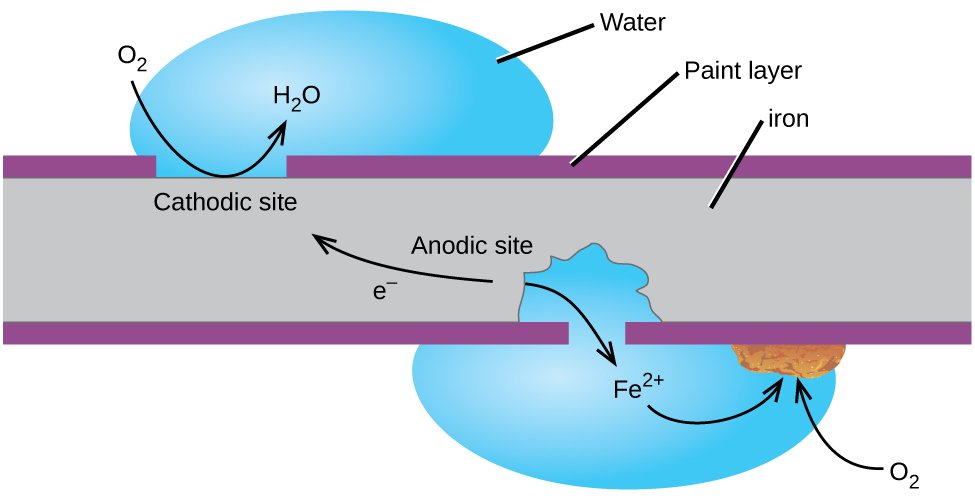
3. Corrosion of Iron
Rusting of iron which is the most commonly seen example happens when iron comes in contact with air or water. The reaction could be seen as a typical electrochemical cell reaction. Consider the diagram given below.
Here metal iron loses electrons and gets converted to Fe2+ . The electrons lost will move to the other side where they combine with H+ ions. H+ ions are released either by H2O or by H2CO3 present in the atmosphere.
- Corrosion of Zinc when it reacts with oxygen and HCl to form white coloured ZnCl2.
- Corrosion of Tin to form black coloured Na2.
You May Like: Geometry Segment Addition Postulate Worksheet
Corrosive Versus Caustic Or Irritant
The term “caustic” is often considered synonymous with “corrosive.” However, only strong bases should be referred to as caustic. Examples of caustic chemicals include sodium hydroxide and potassium hydroxide.
A dilute corrosive chemical acts as an irritant. However, at higher concentrations, corrosive chemicals produce a chemical burn.
While corrosive chemicals may be poisonous, the two characteristics are separate. A poison is a substance with a systemic toxic effect. Poisons may take some time to act. In contrast, a corrosive substance causes an immediate effect on tissue or a surface.
Corrosion Types And Prevention
In a previous post, we discussed the basics of corrosion — from the fundamental chemical reaction to the types of environments in which corrosion can occur. As corrosion most often occurs in aqueous environments, we now explore the different types of degradation a metal can experience in such conditions:
Don’t Miss: Example Of Span Linear Algebra
Examples Of Corrosive Substances
Strong acids and bases are commonly corrosive, although there are some acids that are very powerful, yet not corrosive. Weak acids and bases may be corrosive if they are concentrated. Classes of corrosive substances include:
- strong acids – Examples include nitric acid, sulfuric acid, and hydrochloric acid
- concentrated weak acids – Examples include concentrated acetic acid and formic acid.
- strong Lewis acids – These include boron trifluoride and aluminum chloride
- strong bases – These are also known as alkalis. Examples include potassium hydroxide, sodium hydroxide, and calcium hydroxide.
- alkali metals – These metals and the hydrides of the alkali and alkaline earth metals act as strong bases. Examples include sodium and potassium metal.
- dehydrating agents – Examples include calcium oxide and phosphorus pentoxide.
- strong oxidizers – A good example is hydrogen peroxide.
- halogens – Examples include elemental fluorine and chlorine. The halide ions are not corrosive, except for fluoride.
- acid anhydrides
- organic halides – An example is acetyl chloride.
- alkylating agents – An example is dimethyl sulfate.
- certain organics – An example is phenol or carbolic acid.