Pka And Buffer Capacity
In addition to using pKa to gauge the strength of an acid, it may be used to select buffers. This is possible because of the relationship between pKa and pH:
pH = pKa+ log10
Where the square brackets are used to indicate the concentrations of the acid and its conjugate base.
The equation may be rewritten as:
Ka/ = /
This shows that pKa and pH are equal when half of the acid has dissociated. The buffering capacity of a species or its ability to maintain pH of a solution is highest when the pKa and pH values are close. So, when selecting a buffer, the best choice is the one that has a pKa value close to the target pH of the chemical solution.
Gas Constant In Chemistry
- In chemistry, the gas constant goes by many names, including the ideal gas constant and universal gas constant.
- It is the molar equivalent to the Boltzmann constant.
- The SI value of the gas constant is exactly 8.31446261815324 JK1mol1. Usually, the decimal is rounded to 8.314.
The Gas Constant is the physical constant in the equation for the Ideal Gas Law:
P is pressure, V is volume, n is the number of moles, and T is temperature. Rearranging the equation, you can solve for R:
R = PV/nT
The gas constant is also found in the Nernst equation relating the reduction potential of a half-cell to the standard electrode potential:
E is the cell potential, E0 is the standard cell potential, R is the gas constant, T is the temperature, n is the number of mole of electrons exchanged, F is Faraday’s constant, and Q is the reaction quotient.
The gas constant is equivalent to the Boltzmann constant, just expressed in units of energy per temperature per mole, while the Boltzmann constant is given in terms of energy per temperature per particle. From a physical standpoint, the gas constant is a proportionality constant that related the energy scale to the temperature scale for a mole of particles at a given temperature.
Units for the gas constant vary, depending on other units used in the equation.
What Causes Gas Pressure
Gas pressure is mainly caused due to the collisions that occur between the gas atoms and wall of the container that it is stored in. The atoms move around or travel in the confined space in all directions. During this, the molecules of gas ricochet off the walls gaining momentum and they start exerting some force.
Recommended Reading: Math Magic Tricks With Algebra
Predict Equilibrium And Strength Of Acids Using Ka And Pka
Ka can be used to calculate the equilibrium position:
- When Ka is big, the creation of dissociation products is encouraged.
- When Ka is low, the undissolved acid takes precedence.
Ka can be used to estimate an acids strength:
- If Ka is high , the acid is largely dissociated and therefore powerful. Strong acids have a pKa less than or equal to -2.
- When Ka is low , there has been little dissociation, hence the acid is weak. Weak acids have a pKa in water that ranges from -2 to 12.
Because adding water to an acid solution does not change its acid equilibrium constant, but it does modify the H+ ion concentration and pH, Ka is a better estimate of an acids strength than pH.
Hectopascal And Millibar Units
The units of atmospheric pressure commonly used in meteorology were formerly the bar, which was close to the average air pressure on Earth, and the millibar. Since the introduction of SI units, meteorologists generally measure pressures in hectopascals unit, equal to 100 pascals or 1 millibar. Exceptions include Canada, which uses kilopascals . In many other fields of science, prefixes that are a power of 1000 are preferred, which excludes the hectopascal from use.
Many countries also use millibars. In practically all other fields, the kilopascal is used instead.
Read Also: What Happened To Beth Thomas Biological Father
Understanding Kb And Pkb
Kb is the base dissociation constant. The base dissociation constant is a measure of how completely a base dissociates into its component ions in water.
A large Kb value indicates the high level of dissociation of a strong base. A lower pKb value indicates a stronger base.
pKa and pKb are related by the simple relation:
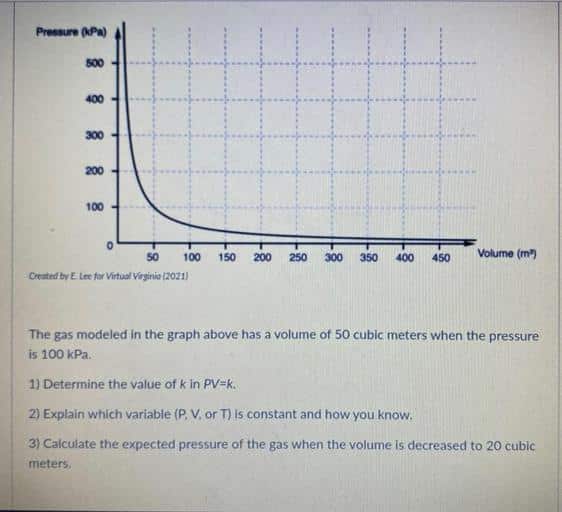
Examples Of Boyles Law
When a filled balloon is squeezed, the volume occupied by the air inside the balloon decreases. This is accompanied by an increase in the pressure exerted by the air on the balloon, as a consequence of Boyles law. As the balloon is squeezed further, the increasing pressure eventually pops it. An illustration describing the increase in pressure that accompanies a decrease in the volume of a gas is provided below.
Examples of Boyles Law
If a scuba diver rapidly ascends from a deep zone towards the surface of the water, the decrease in the pressure can cause the gas molecules in his/her body to expand. These gas bubbles can go on to cause damage to the divers organs and can also result in death. This expansion of the gas caused by the ascension of the scuba diver is another example of Boyles law. Another similar example can be observed in the deep-sea fish that die after reaching the surface of the water .
Also Check: What Is The Entrance Exam For Psychology
The Ideal Gas Constant
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Chemistry and physics equations commonly include “R”, which is the symbol for the gas constant, molar gas constant, ideal gas constant, or universal gas constant. It is a proportionality factor that relates energy scales and temperature scales in several equations.
Why Boyles Law Is Not Applicable At High Pressure
To learn more about Boyles law and other important gas laws, such as Charles law, register with BYJUS, and download the mobile application on your smartphone.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Don’t Miss: What Is The Branch Of Biology Called
Units And The Molar Gas Law
When using the molar gas law, PV = nRT, you have some choices of units. The SI unit of volume is the cubic meter , but that unit can be cumbersome, and the use of liters as a unit of gas volume is often favored. Likewise, the SI unit of pressure is the Pascal , but the atmosphere is used more frequently in some fields
You can adapt to the set of units you’d like to use just by changing the gas constant. Here are the constants and the units of pressure, temperature and volume that go with them. While, when using gas laws like Charles’ law and the Gay-Lussac law, it’s OK to use Celsius temperatures , it’s important to use Kelvin temperatures in the ideal gas law.
R = 8.314 J·mol-1K-1
What Are The Units Used For The Ideal Gas Law
The equation for the Ideal Gas Law is:
Explanation:
On the whole, this is an easy equation to remember and use.
The problems lie almost entirely in the units.
SI units
Pressure is measured in pascals sometimes expressed as newtons per square metre . These mean exactly the same thing.
Be careful if you are given pressures in kilopascals ( #”kPa”# #”150 kPa = 150 000 Pa”# . You must make that conversion before you use the ideal gas law.
The bar is almost an SI unit.
#”1 bar = 100 kPa = 100 000 Pa”#
Volume, #”V”#
This is one place for you to go wrong when you use the Ideal Gas Law.
That’s because the SI base unit of volume is the cubic metre ( #”m”^3# .
#”1 m”^3 = “1000 dm”^3 = “1000 L” = 10^6 “cm”^3 = 10^6 “mL”#
Thus, if you are inserting values of volume into the equation, you first have to convert them into cubic metres.
Number of moles,
This is easy, of course the units are #”mol”#
You will usually be given the value for #R#
#R# is
#R = “8.314 Pa·m”^3·”K”^”-1″”mol”^”-1″ = “8.314 J·K”^”-1″”mol”^”-1″ = “8.314 kPa·dm”^3·”K”^”-1″”mol”^”-1″ =” 8.314 kPa·L·K”^”-1″”mol”^”-1″#
The temperature,
The temperature has to be in kelvins.
Don’t forget to add 273.15 if you are given a Celsius temperature.
ALWAYS make sure that the units you use for #R# .
Non-SI units
The major difference will be that the pressure is given in atmospheres or millimetres of mercury or bars or millibars, and volume may be in litres or millilitres.
If you have to convert from other pressure measurements:
Recommended Reading: Is Sakura Sarada’s Biological Mother
Understanding Ka And Pka
Ka, pKa, Kb, and pKb are most helpful when predicting whether a species will donate or accept protons at a specific pH value. They describe the degree of ionization of an acid or base and are true indicators of acid or base strength because adding water to a solution will not change the equilibrium constant. Ka and pKa relate to acids, while Kb and pKb deal with bases. Like pH and pOH, these values also account for hydrogen ion or proton concentration or hydroxide ion concentration .
Ka and Kb are related to each other through the ion constant for water, Kw:
Ka is the acid dissociation constant. pKa is simply the -log of this constant. Similarly, Kb is the base dissociation constant, while pKb is the -log of the constant. The acid and base dissociation constants are usually expressed in terms of moles per liter . Acids and bases dissociate according to general equations:
- HA + H2O â A- + H3O+
In the formulas, A stands for acid and B for base.
- at half the equivalence point, pH = pKa = -log Ka
A large Ka value indicates a strong acid because it means the acid is largely dissociated into its ions. A large Ka value also means the formation of products in the reaction is favored. A small Ka value means little of the acid dissociates, so you have a weak acid. The Ka value for most weak acids ranges from 10-2 to 10-14.
The pKa gives the same information, just in a different way. The smaller the value of pKa, the stronger the acid. Weak acids have a pKa ranging from 2-14.
Molar Volume Of A Gas
It is equally as important to indicate the applicable reference conditions of temperature and pressure when stating the molar volume of a gas as it is when expressing a gas volume or volumetric flow rate. Stating the molar volume of a gas without indicating the reference conditions of temperature and pressure has very little meaning and can cause confusion.
The molar volume of gases around STP and at atmospheric pressure can be calculated with an accuracy that is usually sufficient by using the ideal gas law. The molar volume of any ideal gas may be calculated at various standard reference conditions as shown below:
- Vm = 8.3145 × 273.15 / 101.325 = 22.414 dm3/mol at 0 °C and 101.325 kPa
- Vm = 8.3145 × 273.15 / 100.000 = 22.711 dm3/mol at 0 °C and 100 kPa
- Vm = 8.3145 × 288.15 / 101.325 = 23.645 dm3/mol at 15 °C and 101.325 kPa
- Vm = 8.3145 × 298.15 / 101.325 = 24.466 dm3/mol at 25 °C and 101.325 kPa
- Vm = 8.3145 × 298.15 / 100.000 = 24.790 dm3/mol at 25 °C and 100 kPa
- Vm = 10.7316 × 519.67 / 14.696 = 379.48 ft3/lbmol at 60 °F and 14.696 psi
- Vm = 10.7316 × 519.67 / 14.730 = 378.61 ft3/lbmol at 60 °F and 14.73 psi
Technical literature can be confusing because many authors fail to explain whether they are using the ideal gas constantR, or the specific gas constant Rs. The relationship between the two constants is Rs = R / m, where m is the molecular mass of the gas.
Read Also: How To Solve Geometry Problems Step By Step
Can You Guess How Old This Car Is
There are several benefits to maintaining the proper air pressure in a car tire. The ride is smoother and safer than it is with too low of pressure. The car gets better gas mileage and the tires do not wear as fast. The recommended pressure for that model of car -\) is generally listed in the owner’s manual or stamped somewhere inside the door. The pressure on the tire is the maximum pressure for that tire, not the recommended one. Tire pressure is best measured when the tire is cold, since driving the car will heat up the air in the tire and increase the pressure.
What Does The P Mean
Whenever you see a “p” in front of a value, like pH, pKa, and pKb, it means you’re dealing with a -log of the value following the “p”. For example, pKa is the -log of Ka. Because of the way the log function works, a smaller pKa means a larger Ka. pH is the -log of hydrogen ion concentration, and so on.
Don’t Miss: Geometry 2nd Grade Common Core Worksheets
How To Calculate Kpa
Engineers often measure or calculate pressure in metric units. The unit for pressure is the Pascal, or one newton of force per square meter of area. Converting pressure to kiloPascals , which equals 1,000 Pascals, will abbreviate large pressure values. You must only consider the amount of force acting perpendicular to the surface. The kPa is also the unit of normal, or axial, stress and shear, or tangential stress. Calculating stress or pressure is a matter of determining the correct force vector and the correct cross-sectional area.
Write all the information you have for your problem on the paper. For a three-dimensional problem, you should have a force vector and some definition for the object you are analyzing at a minimum. If possible, draw a sketch of the problem. In the example, the object is a cylinder with a radius of 0.5m. The force is 20 kilonewtons acting at the center of the top surface at a 30 degree angle from perpendicular. The source is the top surface, which is flat and perpendicular to the centerline of the cylinder.
Convert the force vector into its axial and tangential components. The conversions for this example are: Axial = F = F_cos = 20_cos = 17.3 kN Tangential = F = F_sin = 20_sin = 10 kN
Calculate the cross sectional area perpendicular to the axial component. In this example: A = _r^2 = _0.5^2 = 0.785 m^2
Divide the axial force by the cross-sectional area. P = F/A = 17.3 N / 0.785 m^2 = 22.04 kPa
Things You’ll Need
What Is The Standard Atmospheric Pressure In Kpa
What is the standard atmospheric pressure in kPa? Normal atmospheric pressure at sea level is about 101,000 Pa namely 101 kPa .
Is 1 atm a standard pressure?
Standard atmosphere
| other metric units | 1.013250 bar |
What is standard atmospheric pressure and temperature? U.S. Standard Atmosphere is defined as temperature of 288.15 K at a sea level 0 kilometer geopotential height and pressure of 1 atm .
What is high atmospheric pressure? A barometric reading over 30.20 inHg is generally considered high, and high pressure is associated with clear skies and calm weather.
Also Check: Glencoe Geometry Chapter 10 Answers
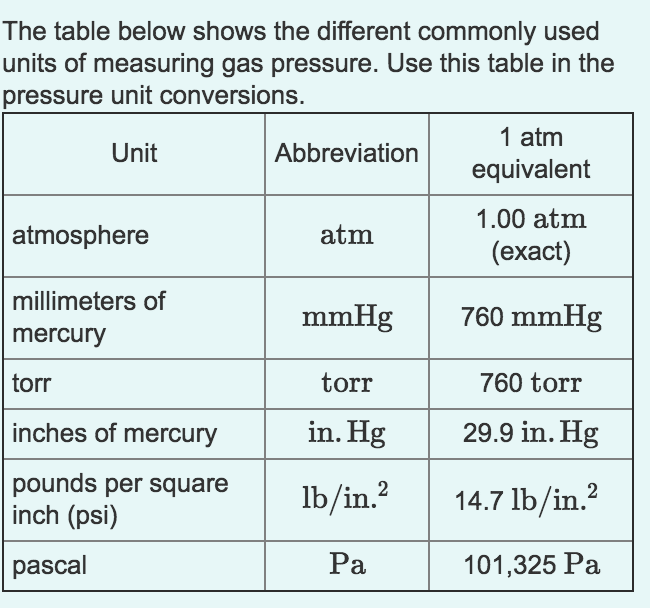
Pressure Units And Conversions
A barometer measures gas pressure by the height of the column of mercury. One unit of gas pressure is the millimeter of mercury \\). An equivalent unit to the \ is called the \, in honor of the inventor of the barometer, Evangelista Torricelli. The pascal \\) is the standard unit of pressure. A pascal is a very small amount of pressure, so the most useful unit for everyday gas pressures is the kilopascal \\). A kilopascal is equal to 1000 pascals. Another commonly used unit of pressure is the atmosphere \\). Standard atmospheric pressure is defined as \ of pressure and is equal to \ and \. Atmospheric pressure is also often stated as pounds per square inch \\). The atmospheric pressure at sea level is \.
It is important to be able to convert between different units of pressure. To do so, we will use the equivalent standard pressures shown above.
Example \: Pressure Unit Conversions
The atmospheric pressure in a mountainous location is measured to be \. What is this pressure in \ and in \?
Solution
Use conversion factors from the equivalent pressure units to convert from \ to \ and from \ to \.
Step 2: Solve.
Step 3: Think about your result.
The air pressure is about \ of standard atmospheric pressure at sea level. For significant figure purposes, the standard pressure of \ has three significant figures.
Acid Dissociation Constant From Ph
The pH scale, or power of hydrogen, is a numerical measure of a solutions acidity or basicity. In an aqueous solution, it can be used to compute the concentration of hydrogen ions or hydronium ions . Low pH solutions are the most acidic, whereas high pH solutions are the most basic.
The concentration of free hydrogen ions in an aqueous acid solution is measured by its pH: pH equals -log or -log . The last equation can be rewritten as follows:
The above comparison allows you to determine the relative concentration of acid to conjugate base and estimate the dissociation constant Ka if you know the molar concentration of an acid solution and can detect its pH.
Recommended Reading: How To Calculate Ihd Organic Chemistry
What Is A Good Example Of Boyles Law
A balloon is a good example of Boyles law in action. The balloon is inflated by blowing air into it the pressure of the air pulls on the rubber, causing the balloon to expand. When one end of the balloon is compressed, the pressure within rises, causing the un-squeezed section of the balloon to expand outward.
Kpa To Psi Pressure Conversion: How To Convert Kilopascals To Psi
By rggroup on April 14th, 2019 in General
Pressure is defined as a force exerted on a surface per unit area. To put it more simply, its the force that is put on a certain defined area.
Youre probably most familiar with the concept as we use it in everyday life. For example, a doctor might say, apply pressure to the wound, in which case you would press on the wound .
But youve also heard this in a scientific and/or industrial context with air pressure, tire pressure, water pressure, etc. Often, youll see the units of pressure change depending on the context. Kilopascals and pound-force per square inch are the two most common.
Sometimes, youll need to switch between those units. In this article, were going to take you through exactly how to go from kPa to psi, when you need to do that, along with more information on each of the units.
You May Like: What Is Internal Transport In Biology