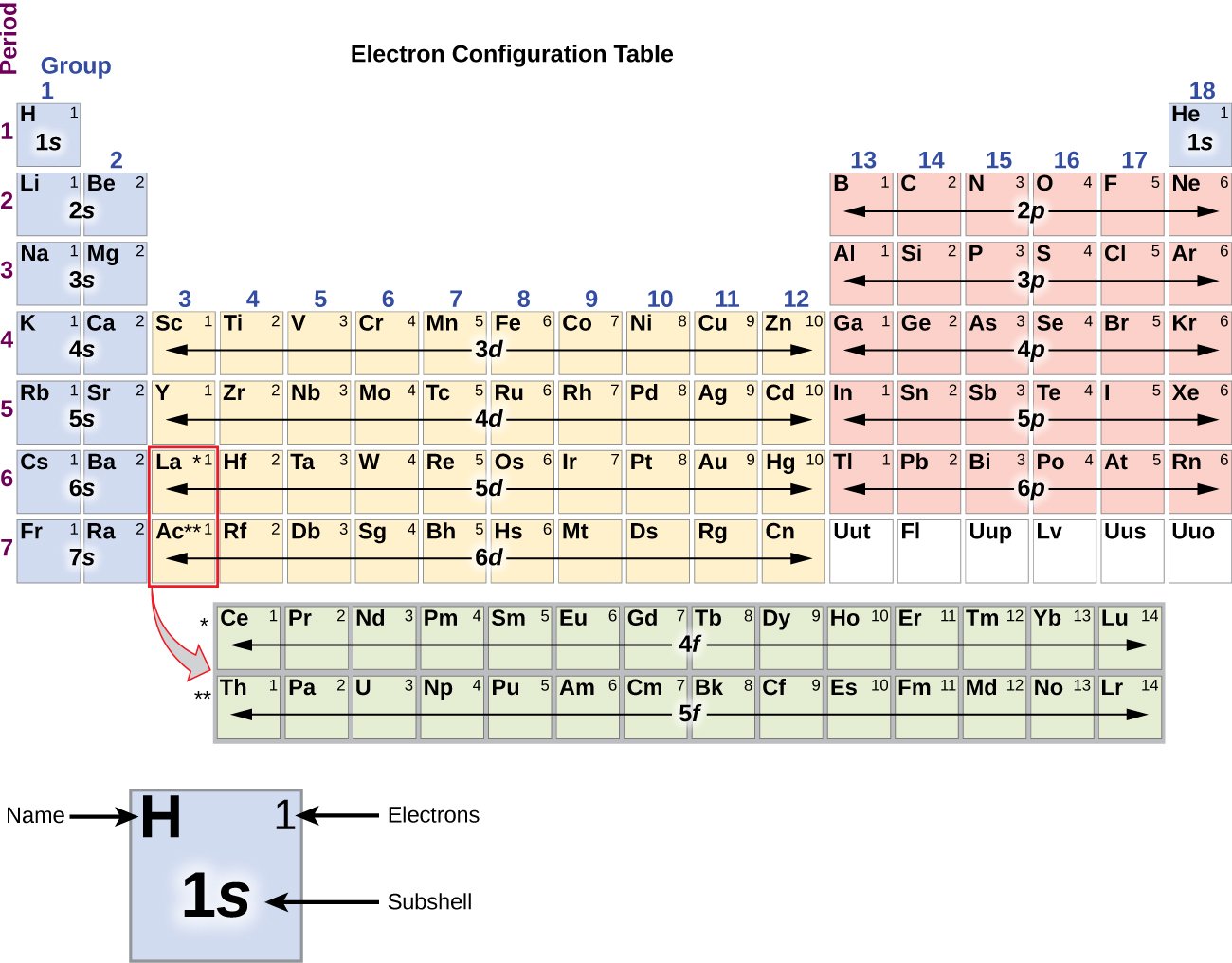
Definition Of Electron Configuration
The electronic configuration is the arrangement with which the;electrons;are arranged or organized;in an atom based on the model of electronic shells;.;In this way, the;properties;in which the;elements are;chemically combined;can be explained;.;Under this principle the;order;was established;in the;modern;periodic table;.
What Is Meant By The Electronic Configuration Of An Element
The electronic configuration of an element is a symbolic notation of the manner in which the electrons of its atoms are distributed over different atomic orbitals. While writing electron configurations, a standardized notation is followed in which the energy level and the type of orbital are written first, followed by the number of electrons present in the orbital written in superscript. For example, the electronic configuration of carbon is 1s22s22p2.
Important Principle Use While Write Electronic Configuration Of Elements
Paulis Exclusion principle
According to this principle, an orbital can contain a maximum number of two electrons and these two electrons must be of opposite spin.
Two electrons in an orbital can be represented by ||;
Hunds rule of maximum multiplicity
This rule deals with the filling of electrons in the equal energy orbitals of the same sub shell . According to this rule,
Electron pairing in p,d and f orbitals cannot occur until each orbital of a given subshell contains one electron each or is singly occupied & that too with the same spin.
This is due to the fact that electrons being identical in charge, repel each other when present in the same orbital. This repulsion can, however, be minimized if two electrons move as far apart as possible by occupying different degenerate orbitals. All the electrons in a degenerate set of orbitals will have same spin.
Multiplicity is given by 2|S| + 1. Can you now comment why the rule is called Hunds rule of maximum multiplicity.
You May Like: Lesson 9.4 Practice B Geometry Answers
Electronic Configuration In Periods And Groups
The electronic configuration of an atom is the numerical representation of the arrangement of electrons distributed in the orbitals of the atom. This determines the position of an element in the periodic table and in turn its chemical behavior. It explains how the atoms are held together by the chemical bonds, and the peculiar trends which are observed in the rows and columns of the periodic table. In this article, we will discuss the electronic configuration of elements in the same periods and groups of the periodic table.
Electronic Configuration in Periods
Electronic Configuration in Groups
Elements in the same group have the same number of electrons in their outermost shell leading to similar valence shell electronic configuration. Thus, we observe a similar trend in the properties and chemistry of the elements in the same group.
To learn more about electronic configuration and periodic trends with the vibrant video lectures, download BYJUâS â The Learning App.
Put your understanding of this concept to test by answering a few MCQs. Click âStart Quizâ to begin!
Select the correct answer and click on the âFinishâ buttonCheck your score and answers at the end of the quiz
Electron Configuration In Molecules
In molecules, the situation becomes more complex, as each molecule has a different orbital structure. The molecular orbitals are labelled according to their symmetry, rather than the atomic orbital labels used for atoms and monatomic ions: hence, the electron configuration of the dioxygen molecule, O2, is written 1g2;1u2;2g2;2u2;3g2;1u4;1g2, or equivalently 1g2;1u2;2g2;2u2;1u4;3g2;1g2. The term 1g2 represents the two electrons in the two degenerate *-orbitals . From Hund’s rules, these electrons have parallel spins in the ground state, and so dioxygen has a net magnetic moment . The explanation of the paramagnetism of dioxygen was a major success for molecular orbital theory.
The electronic configuration of polyatomic molecules can change without absorption or emission of a photon through vibronic couplings.
Read Also: Fsa Algebra 1 Eoc Review Functions And Modeling Answer Key
Purpose Of Electron Configurations
When atoms come into contact with one another, it is the outermost electrons of these atoms, or valence shell, that will interact first. An atom is least stable when its valence shell is not full. The valence electrons are largely responsible for an elements chemical behavior. Elements that have the same number of valence electrons often have similar chemical properties.
Electron configurations can also predict stability. An atom is at its most stable when all its orbitals are full. The most stable configurations are the ones that have full energy levels. These configurations occur in the noble gases. The noble gases are very stable elements that do not react easily with any other elements.
Electron configurations can help to make predictions about the ways in which certain elements will react and the chemical compounds or molecules that different elements will form. These principles help to understand the behavior of all chemicals, from the most basic elements like hydrogen and helium, to the most complex proteins found in the human body.
Principle Of The Aufbau
It is possible to write the electronic configuration of all elements based on the energies sequence for the orbitals and the Pauli Exclusion Principle.
This procedure is based on the Aufbau principle, making it possible to construct the periodic table of the elements by determining their electronic configuration progressively.
Each step requires adding an electron to the appropriate orbital.
After helium, the next element in the periodic table is lithium, which has three electrons.
Due to the Pauli Exclusion Principle, an orbital cannot host more than one electron, so the third electron cannot stay in the 1s orbital:
It will have to occupy the next orbital with the lowest possible energy. Therefore, the electron configuration of lithium is 1s2, 2s1.
In a similar way, we obtain the electronic configuration of beryllium, which has four electrons as 1s2, 2s2.
Now both the 1s and 2s orbitals are fully occupied, while the next element, boron, has five electrons and its extra electron must be positioned in the 2p sublevel as all three 2p sublevels have the same energy . It can indifferently occupy any of them.
You May Like: Geometry Segment Addition Postulate Worksheet
Valency And Valence Electrons
The outermost shell of an atom is its valence shell, and the electrons in the valence shell are valence electrons. Valence electrons are the highest energy electrons in an atom and are therefore the most reactive. While inner electrons typically don’t participate in chemical bonding and reactions, valence electrons can be gained, lost, or shared to form chemical bonds. For this reason, elements with the same number of valence electrons tend to have similar chemical properties, since they tend to gain, lose, or share valence electrons in the same way. The Periodic Table was designed with this feature in mind. Each element has a number of valence electrons equal to its group number on the Periodic Table.
The electron configurations for the first and second row elements are shownin in simplified notation.
The N + Energy Ordering Rule
A periodic table in which each row corresponds to one value of n + was suggested by Charles Janet in 1928, and in 1930 he made explicit the quantum basis of this pattern, based on knowledge of atomic ground states determined by the analysis of atomic spectra. This table came to be referred to as the left-step table. Janet “adjusted” some of the actual n + values of the elements, since they did not accord with his energy ordering rule, and he considered that the discrepancies involved must have arisen from measurement errors. In the event, the actual values were correct and the n + energy ordering rule turned out to be an approximation rather than a perfect fit, although for all elements that are exceptions the regularised configuration is a low-energy excited state, well within reach of chemical bond energies.
In 1936, the German physicist Erwin Madelung proposed this as an empirical rule for the order of filling atomic subshells, and most English-language sources therefore refer to the Madelung rule. Madelung may have been aware of this pattern as early as 1926.In 1945 William Wiswesser proposed that the subshells are filled in order of increasing values of the function
- W . }.}
In addition to there being ample experimental evidence to support this view, it makes the explanation of the order of ionization of electrons in this and other transition metals more intelligible, given that 4s electrons are invariably preferentially ionized.
You May Like: Algebra 1 Eoc Answers 2015
How To Write An Electron Configuration
The symbols used for writing the electron configuration start with the shell number followed by the type of orbital and finally the superscript indicates how many electrons are in the orbital.
For example:
Looking at the periodic table, you can see that Oxygen has 8 electrons. Based on the order of fill above, these 8 electrons would fill in the following order 1s, 2s and then 2p. So Oxygen’s electron configuration would be O 1s22s22p4.
What Is The Electronic Configuration Of Copper
The electronic configuration of copper is 3d104s1. This configuration disobeys the aufbau principle due to the relatively small energy gap between the 3d and the 4s orbitals. The completely filled d-orbital offers more stability than the partially filled configuration.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Read Also: Geometry Lesson 1.7 Answers
What Is Electronic Configuration For
The concept of electron configuration establishes the way in which the ordering of electrons within an atom associated with a given element is determined;.;This allows it to be possible to appreciate at an;atomic;level;everything related to the;properties;of an;element;and the mechanism of its;chemical;reactions;.
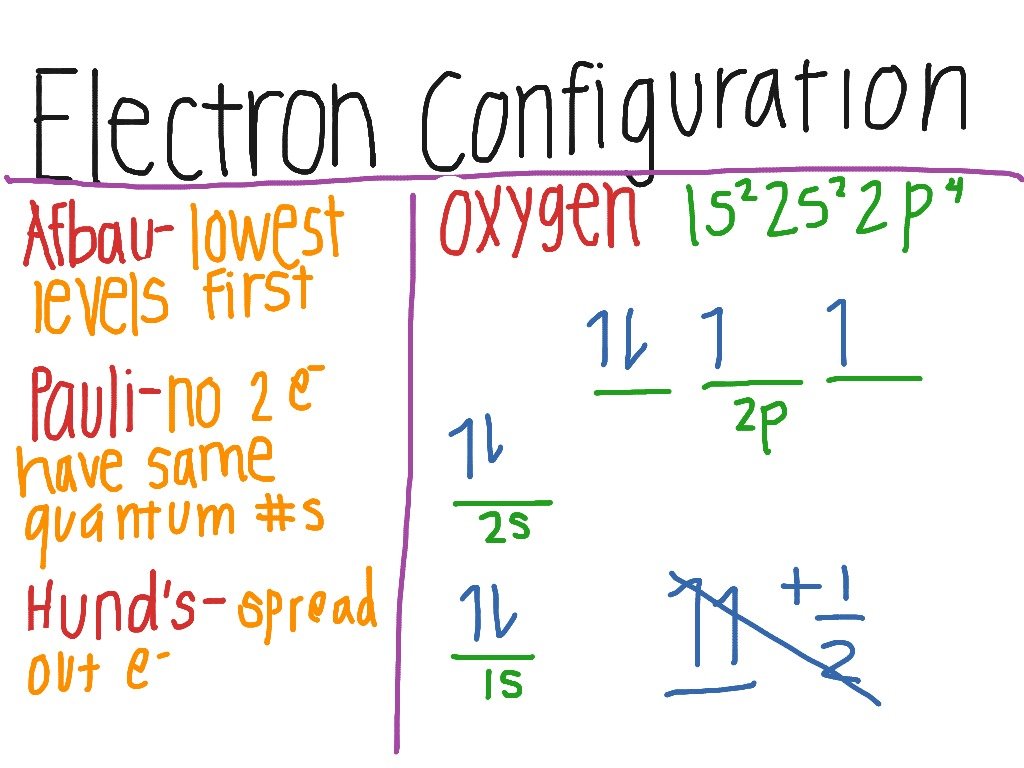
Electron Configuration Of Oxygen
The atomic number of oxygen is 8, implying that an oxygen atom holds 8 electrons. Its electrons are filled in the following order:
K shell 2 electrons
L shell 6 electrons
Therefore, the electron configuration of oxygen is 1s2 2s2 2p4, as shown in the illustration provided below.
Electron Configuration of Oxygen
Also Check: What Are Dyes
What Does Electron Configuration Tell You About An Element
It tells you how many electrons there are in each of the different orbitals and at which energy level .
Examples:
#1s^ -># 2 electrons in the s orbital on first energy level. (this happens to be the electron configuration of helium, #He#
# 2s^ 2p^ -># electron configuration of fluorine, . Here’s how you’d break down the configuration
- #1s^# , so 2 electrons in the s orbital on 1st energy level – this is the electron configuration of helium;
- #2s^# , so 2 electrons in s orbital on 2nd energy level;
- #2p^#
What Are The Three Rules That Must Be Followed While Writing The Electronic Configuration Of Elements
The three rules that dictate the manner in which electrons are filled in atomic orbitals are:
- The Aufbau principle: electrons must completely fill the atomic orbitals of a given energy level before occupying an orbital associated with a higher energy level. Electrons occupy orbitals in the increasing order of orbital energy level.
- Paulis exclusion principle: states that no two electrons can have equal values for all four quantum numbers. Consequently, each subshell of an orbital can accommodate a maximum of 2 electrons and both these electrons MUST have opposite spins.;
- Hunds rule of maximum multiplicity: All the subshells in an orbital must be singly occupied before any subshell is doubly occupied. Furthermore, the spin of all the electrons in the singly occupied subshells must be the same .
Also Check: What Type Of Math Is On The Ged
Electron Configuration With Examples
Electron Configuration with Examples
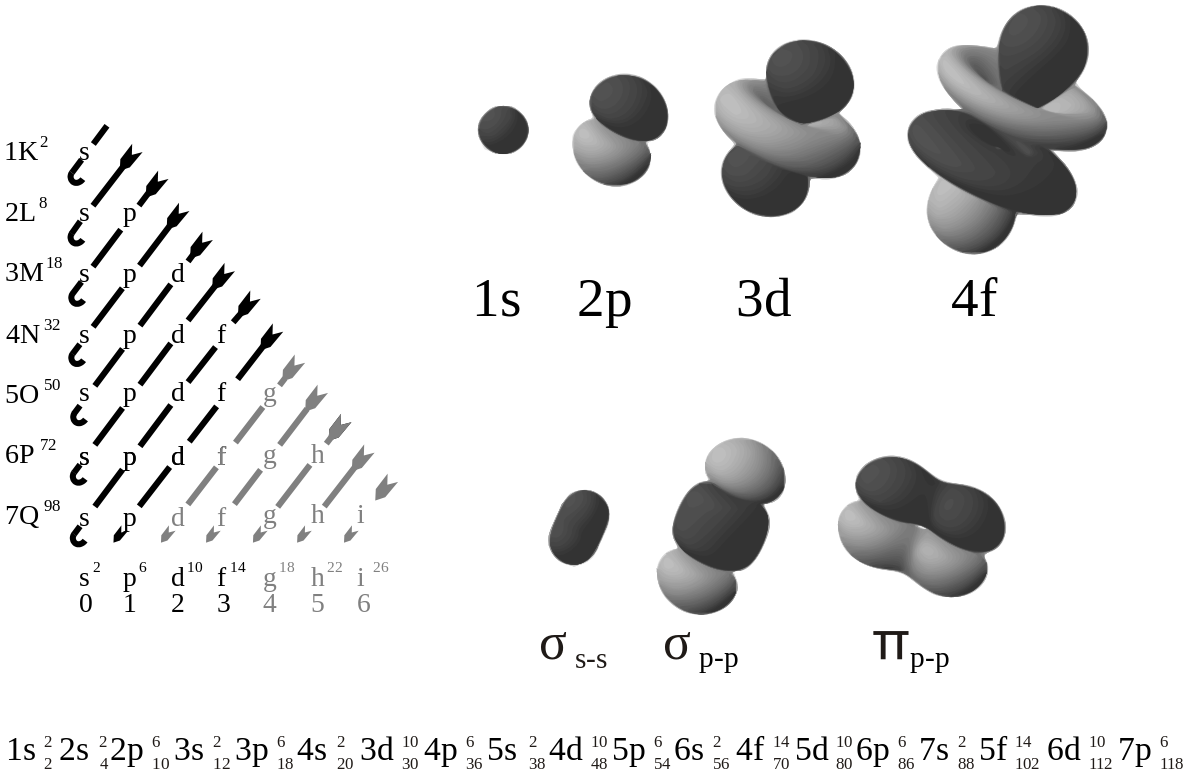
Electrons are not placed at fixed positions in atoms, but we can predict approximate positions of them. These positions are called energy levels or shells of atoms.
- Lowest energy level is 1 and it is denoted with integer n=1, 2, 3, 4, 5, 6… or letters starting from K, L, N to Q.; An atom can have maximum 7 energy levels and electrons can change their levels according to their energies.
- Each energy level has different number of electrons. For example, we can find number of electrons in four energy level with following formula; 2n2.
1st energy level has;
3rd energy level has;
2n2=2.32=18 electrons
- Electrons are located energy levels starting from the first energy levels. If one of the energy level is full, then electrons are placed following energy level.
Following pictures show location of electrons of atoms O and Mg.
Number of electrons at the outer shell of atom gives us following classification.
Electron configuration of atom shows, shells, sub shells and number of electrons in sub shells. We examine electron configuration with following examples.
Example: Helium 2
1 is the principal quantum number or energy level
s is the sub-level or sub shell
2 shows the number of electrons in the s sub shell
Example: Chlorine 17
1s22s22p63s23p5
Coefficients 1, 2, 2, 3, and 3 are energy levels of Cl. As you can see “p” sub shell can have maximum 6 electrons.
Superscripts 2, 2, 6, 2 and 5 are electrons in the sub shells “s” and “p”.
Open And Closed Shells
In the context of atomic orbitals, an open shell is a valence shell which is not completely filled with electrons or that has not given all of its valence electrons through chemical bonds with other atoms or molecules during a chemical reaction. Conversely a closed shell is obtained with a completely filled valence shell. This configuration is very stable.
For molecules, “open shell” signifies that there are unpaired electrons. In molecular orbital theory, this leads to molecular orbitals that are singly occupied. In computational chemistry implementations of molecular orbital theory, open-shell molecules have to be handled by either the restricted open-shell HartreeFock method or the unrestricted HartreeFock method. Conversely a closed-shell configuration corresponds to a state where all molecular orbitals are either doubly occupied or empty . Open shell molecules are more difficult to study computationally
Don’t Miss: Geometry Segment Addition Postulate Worksheet
Writing The Electron Configurations Examples
Finding carbons electron configuration
Lets start with finding the electron configuration for carbon . First, we need to know how many electrons carbon has. Since carbon has the atomic number six, it will also have six protons and six electrons. Furthermore, carbon is located in the second period and the second group in block p. When finding electron configurations, we will work left to right. The corresponding electron shell configuration is, therefore: 1s22s22p2. The superscripts represent the number of electrons present and can be added to find the total number of electrons in an atom. To help visualize, this is how each subshell looks with the six electrons.
Finding phosphorus
Now, lets find phosphorus on the periodic table. It is located in the third period and within the p-block. Moreover, it has fifteen electrons. The electron shell configuration for phosphorous would therefore be 1s22s22p63s23p3.
Filling In An Aufbau Diagram
The order in which orbitals are filled is given by the Madelung rule. The rule is based on the total number of nodes in the atomic orbital, n + , which is related to the energy. In this context, n represents the principal quantum number and represents the azimuthal quantum number. The values = 0, 1, 2, 3 correspond to the s, p, d, and f labels, respectively. According to the principle, electrons fill orbitals starting at the lowest available energy states before filling higher states .
The Madelung energy ordering rule: Order in which orbitals are arranged by increasing energy according to the Madelung Rule. Each diagonal read arrow corresponds to a different value of n + l.
An Aufbau diagram uses arrows to represent electrons. When there are two electrons in an orbital, the electrons are called an electron pair. Electron pairs are shown with arrows pointing in opposite directions. According to the Pauli Exclusion Principle, two electrons in an orbital will not spin the same way. That is, an Aufbau diagram uses arrows pointing in opposite directions. An arrow pointing up denotes an electron spinning one way and an arrow pointing downwards denotes an electron spinning the other way. If the orbital only has one electron, this electron is called an unpaired electron.
Aufbau diagram for lithium: The electron configuration of lithium, shown on an Aufbau diagram
The following steps detail how to draw an Aufbau diagram:
Also Check: What Is The Molecular Geometry Of Ccl4
Electronic Configuration Of Chromium Atom
The atomic number of Chromium = 24
So, lets start writing the electronic configuration of Chromium based upon the Aufbau Principle. According to Aufbau Principle, the orbitals are filled up with electrons in order of their increasing energy, i.e., orbital of lowest energy are filled up first, and those with the highest energy are filled up at the end.
- According to Aufbau Principle, the first two electrons of Chromium are filled in 1s orbital.
- Then, the next two electrons for Chromium occupy in the 2s orbital.
- And, next 6 electrons for Chromium occupy in the 2p orbital.
- Then, the next two electrons go in the 3s.
- Further, the next 6 electrons occupy the 3p orbital.
- Now, by following the same rule of the Aufbau Principle, within the remaining 6 electrons, two electrons are placed in 4s orbital, and 4 electrons are placed in the 3d orbital. Thus, the expected electronic configuration of Chromium will be as follows:
The expected electronic configuration of Chromium: 1s22s22p63s23p6;3d4;4s2
However, halffilled;or;fully-filled orbitals;are;more stable;due to the symmetrical distribution of electrons. Therefore, one of the 4s2;electrons of the Chromium atom jumps to the 3d5;to achieve a half-filled 3d orbital. Thus, the correct electronic configuration of the chromium atom will be as follows:
The correct electronic configuration of Chromium: 1s22s22p63s23p6;3d5;4s1
Final Answer
Electronic configuration of Chromium atom : 1s22s22p63s23p6;3d5;4s1
What Is Electron Configuration
In quantum chemistry and atomic physics, the electron configuration of an atom or molecule describes the distribution of electrons in different atomic or molecular orbitals. It also describes every electron as moving freely in an orbital, in an average field generated by other orbitals. For example, electron configuration of Phosphorus is 1s^2 2s^2 2p^6 3s^2 3p^3.
Usually, Physicists and chemists use the standard notation to refer to the electronic configurations of molecules and atoms. For atoms, the standard notation consists of a series of atomic subshell labels , where the number of electrons assigned to each subshell is used as a superscript. For example, hydrogen has just 1 electron in the s orbital of the first shell, so its electron configuration notation is recorded as 1s^1. Lithium has two electrons in the 1s subshell and one electron in the 2s subshell , so its electron configuration is 1s^2 and 2s^1.
Apart from this, Electron Configuration:
- Use for interpreting atomic spectra.
- Finds the valency of an element.
Also Check: Ccl4 Valence Electrons