Definition Of Crystal Lattice
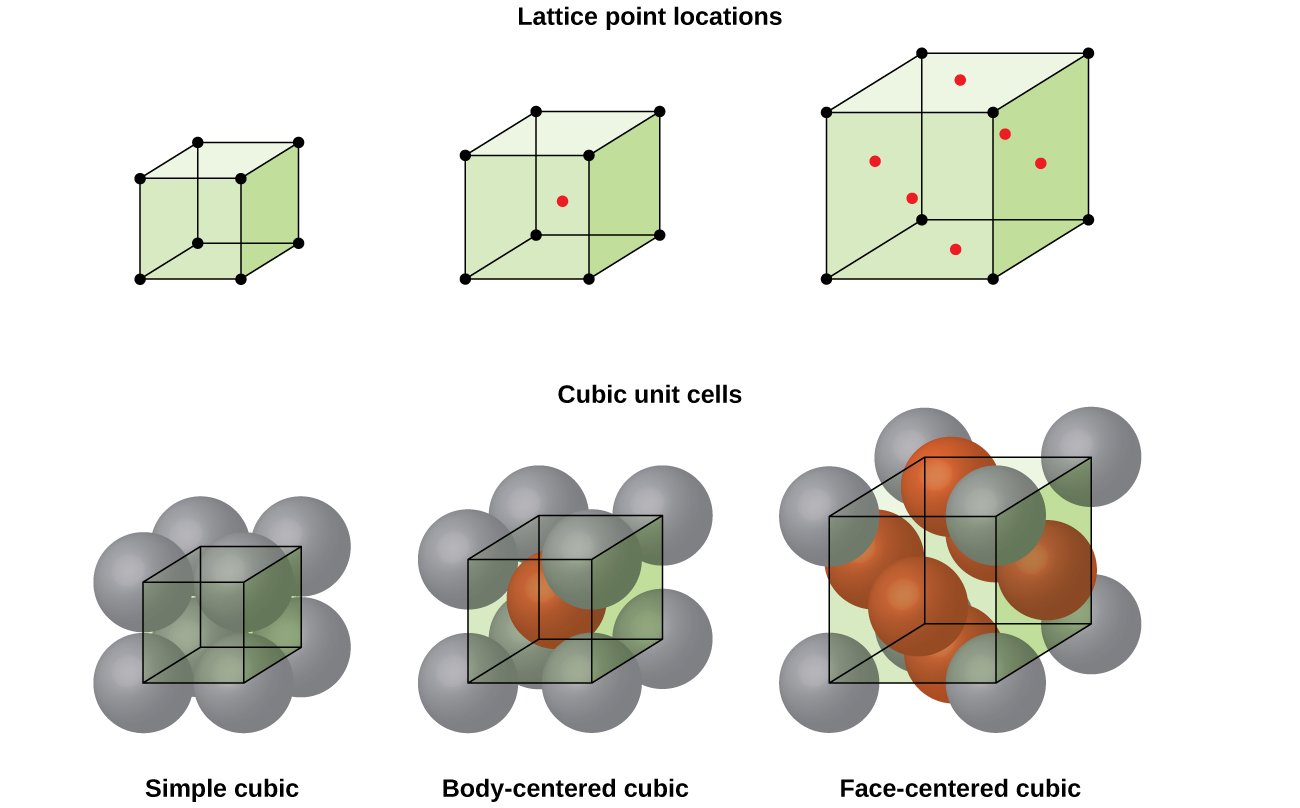
The unit cell of the lattice is the basic repeating unit of the lattice and is characterized by a parallelepiped with cell edge lengths a, b, c and inter axis angles , ,. Bravais Lattices. These unit cells can be classified as belonging to one of fourteen Bravais lattices. Each Bravais lattice belongs to one of the seven crystal systems Lattice energies are also important in predicting the solubility of ionic solids in H 2 O. Ionic compounds with smaller lattice energies tend to be more soluble in H 2 O. Lattice Energies – Chemistry Tutorial. This tutorial covers lattice energy and how to compare the relative lattice energies of different ionic compounds Lattice is a large network of atoms which has an ordered structure. In chemistry, we can see different types of ionic and covalent lattices. We can define a lattice as a solid that has a three- dimensional ordered arrangement of basic units. The basic unit can be an atom, molecule or an ion
LATTICE ENERGIES AND THEIR SIGNIFICANCE IN INORGANIC CHEMISTRY T C Waddington The Uoiversity Chemical laboratories. Cambridge. dispersion energy terms, are included. They obtain for the lattice energy UO The values and meaning of C and D and hv, will be discussed in the next section Chemistry Dictionary. Definition of Crystal Lattice Energy . Amount of energy that holds a crystal together the energy change when a mole of solid is formed from its constituent molecules or ions in their gaseous state
Example : Calculation Of Ionic Radii
The edge length of the unit cell of LiCl is 0.514 nm or 5.14 Å. Assuming that the lithium ion is small enough so that the chloride ions are in contact, as in Figure 15, calculate the ionic radius for the chloride ion.
Note: The length unit angstrom, Å, is often used to represent atomic-scale dimensions and is equivalent to 1010 m.
On the face of a LiCl unit cell, chloride ions contact each other across the diagonal of the face:
Drawing a right triangle on the face of the unit cell, we see that the length of the diagonal is equal to four chloride radii , so d = 4r. From the Pythagorean theorem, we have:
}^+}^=}^
which yields:
\left^+\left^=^=16^
Solving this gives:
r=\sqrt)}^+)}^}}=\text for a Cl radius
Check Your Learning
The edge length of the unit cell of KCl is 6.28 Å. Assuming anion-cation contact along the cell edge, calculate the radius of the potassium ion. The radius of the chloride ion is 1.82 Å.
It is important to realize that values for ionic radii calculated from the edge lengths of unit cells depend on numerous assumptions, such as a perfect spherical shape for ions, which are approximations at best. Hence, such calculated values are themselves approximate and comparisons cannot be pushed too far. Nevertheless, this method has proved useful for calculating ionic radii from experimental measurements such as X-ray crystallographic determinations.
What Is The Definition Of Crystal Lattice Diagram
Definition of the vocabulary of crystal lattice chemistry. Chemistry Dictionary. Determination of the crystal lattice. What is a trellis? A grid is an ordered grid of dots that describes the arrangement of the particles that make up a crystal. The unit cell of the crystal is determined by the points of the lattice.
Also Check: Holt Geometry Chapter 7 Test Form B Answer Key
What Is Lattice Energy In Chemistry
What is Lattice Energy? Lattice energy is a measure of the strength of the ionic bonds in an ionic compound. It provides insight into several properties of ionic solids including their volatility, their solubility, and their hardness. The lattice energy of an ionic solid cannot be measured directly The energy released in this process is known as lattice energy or lattice enthalpy. That means, energy released when a cation and a anion combine together to form one mole of an ionic compound is know as lattice energy or lattice enthalpy
The lattice energy is the energy released by forming solid ionic compounds from free ions: Gaseous ion pairs have already released a part of the lattice energy. Note that for ion pairs, the pairing energy may be smaller than the sum of IE+EA, especially for big ions, so forming pairs may not be favoured Lattice Energy Explanation Lattice energy is the measurement of the stability of a crystal lattice, provided by the energy that would be released per mole if atoms, ions, or molecules of the crystal were brought together from infinite distances to form the lattice
Lattice Energy Definition Trend Formula And Lattice
20.1 Introduction to Lattice Energy What is lattice energy? In a solid ionic crystal lattice, the ions are bonded by strong ionic bonds between them. These forces are only completely broken when the ions are in gaseous stat In one definition, the lattice energy is the energy required to break apart an ionic solid and convert its component atoms into gaseous ions. This definition causes the value for the lattice energy to always be positive, since this will always be an endothermic reaction The amount of energy liberated in condensing the required number of cations and anions to form the lattice of one gram mole of an ionic compound is called the lattice energy of that compound. We can view lattice energy as a measure of how stable the ionic compound is and how easy it is for the formation of ionic compound to take place
Lattice Energy, for a crystalline solid, is the measure of the energy released when ions are combined to make a compound. We can also describe it as a measure of the cohesive forces that bind ions. It is relevant to many properties such as solubility, hardness, and volatility Chemistry Dictionary. Definition of Crystal Lattice Energy . Amount of energy that holds a crystal together the energy change when a mole of solid is formed from its constituent molecules or ions in their gaseous state
You May Like: Introduction To Exponential Functions Common Core Algebra 1 Homework Answers
Charge Held By The Constituent Ions
Due to the electrostatic forces between them, the individual ions in an ionic lattice are attracted to each other. The strength of the electrostatic force of attraction is directly proportional to the magnitude of the charge held by the constituent ions, i.e. the greater the charge, the stronger the force of attraction, the stronger the lattice.
For example, the lattice energy of calcium chloride is greater than that of potassium chloride despite the similarity in the crystal arrangements of these compounds. This is because the magnitude of the positive charge held by the calcium cation is greater than that held by the potassium cation . As a consequence of this, the electrostatic forces of attraction are stronger in calcium chloride . Therefore, the lattice energy of CaCl2 is greater than that of KCl.
Question: What Is Lattice Constant/ What Is Lattice Parameter
Answer:
The lattice parameters or lattice constant can be defined as the quantities which specify a unit cell. These parameters are of six types. The dimensions along the edges of a unit cell are represented by a, b and c along x, y and z planes respectively and angle between b and c is represented by , angle between a and c by and angle between a and b by .
You May Like: Kuta Software Infinite Geometry The Segment Addition Postulate Answer Key
Chem C: Lattice Energy
What Exactly Is Lattice Energy
- How lattice energy affect boiling point ? CONTENTS. The molar lattice energy of an ionic crystal can be expressed in terms of molar lattice enthalpy, pressure, and change in volume via the following equation: Therefore, the outer pressure is also considered when calculating the lattice energies of ionic solids. The name is a portmanteau o
- Energy required to break 1 mole of crystal lattice into its infinitely spaced gaseous ions
- Lattice energy is a type of potential energy that relates to the stability of ionic solids. Ionic solids are very stable, which means that it takes a lot of energy to break their bonds. One place..
- CHAPTER 20: Lattice Energy. Introduction to Lattice Energy Born-Haber Cycles Ion Polarisation Enthalpy Changes in Solutions Learning outcomes: explain and use the term lattice energy .explain, in qualitative terms, the effect of ionic charge and of ionic radius on the numerical magnitude of a lattice energy
- The lattice energy, U, is the necessary energy to separate ions of a mole of MX as a gaseous ions from each other by infinite distances
- Lattice Energies and the Strength of the Ionic Bond . The force of attraction between oppositely charged particles is directly proportional to the product of the charges on the two objects and inversely proportional to the square of the distance between the objects .The strength of the bond between the ions of opposite charge in an ionic compound therefore depends on the.
Recommended Reading: Practice Workbook Geometry Mcdougal Littell Answers
What Are The Building Blocks Of A Lattice
A grid is a three-dimensional periodic table made up of identical building blocks. The building blocks are atoms or groups of atoms. Crystals often have structural defects and impurities. The periodicity of the crystals is well confirmed by experimental studies of X-ray, neutron and electron diffraction patterns.
Trends Of Lattice Energy In The Periodic Tabl
- Chemistry Chemistry Define the term lattice energy. Why, energetically, do ionic compounds form? Fig. 3-8 illustrates the energy changes involved in the formation of MgO and NaF
- Chemistry Cambridge A2 – Slides of Chapter 1. Topic is Chemistry Lattice Energy
- ation energy for Mg +148 kJ/mol Br 2 —> 2Br +193 kJ/mol First ionization energy of Mg +738 Second.
Read Also: How Old Are Elton Johns Kids
What Are Lattice Points
There are different types of lattices. A lattice point is a point in any of these lattices. Lattices are used to describe highly ordered systems such as crystals and some supersolids. I am unsure whether lattices can only describe periodic systems . However, I will be assuming that we are talking about periodic lattices. I will also be assuming that we are dealing with crystals.
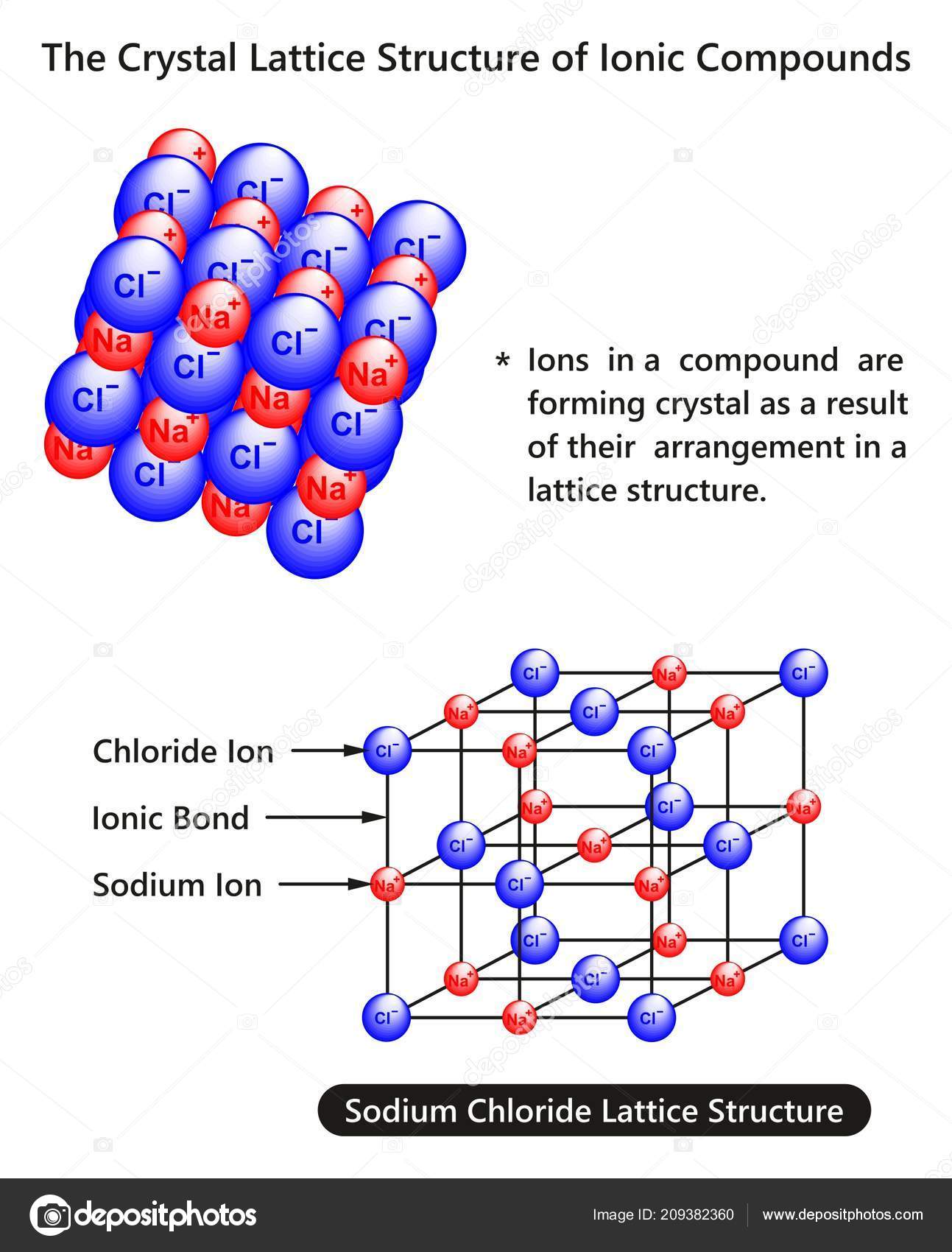
Crystal lattice
The crystal lattice is used to describe the lattice of a real crystal. For example, in NaCl, a lattice point in a crystal lattice represents the position of a sodium ion or a chloride ion.
Bravais lattice
Bravais lattices are more mathematical and abstract than crystal lattices. They are pretty much the same as crystal lattices. Unlike the crystal lattice, however, lattice points in the Bravais lattice no longer represent a position of a particular atom. Instead a lattice point represents a position in which an atom can be placed. In other words, a lattice point in a Bravais lattice is a point, which is equal and indistinguishable from any other another point. What matters in a Bravais lattice are not the points themselves, but how they are arranged .
Reciprocal lattice
Finally, as mentioned by other posts, these points can be vacant.
Distance Between The Ions
The lattice energy of an ionic compound is inversely proportional to the distance between the ions. The further the distance between the ions in a lattice, the weaker the electrostatic forces holding them together, the lower the lattice energy.
Smaller atoms feature smaller interatomic distances in the ionic lattice and stronger binding forces. Therefore, the smaller the size of the constituent ions, the greater the lattice energy of the ionic solid.
Read Also: Is Physics Hard In College
Crystal Lattices And Unit Cells
The crystal lattice is the pattern formed by the points and used to represent the positions of these repeating structural elements. The periodic structure of an ideal crystal is most easily described by a lattice. The crystal lattice is the array of points at the corners of all the unit cells in the crystal structure.
Chemistry End Of Chapter Exercises
What is the atomic radius of tungsten in this structure?
Calculate the density of tungsten.
What is the atomic radius of barium in this structure?
Calculate the density of barium.
Read Also: What Is Elastic Force
Examples: Using Approximation Techniques
First, we will practice solving for the charge variable.
Problem 1: Given the compound MgO, determine its combined charge.
Steps to Solve:
1. Write out the charges of its ions: Mg+2 and O-2
2. Multiply these charges: x = -4
Problem 2: Given the compound KCl, determine its combined charge.
1. Write out the charges of its ions: K+1 and Cl-1
2. Multiply these charges: x = -1
We can compare the -4 charge of MgO to the -1 charge of KCl as discussed. As the former is 4 times the quantity of the latter, we can predict that its lattice energy would be approximately 4 times greater as well.
Now, we will practice solving for the size variable.
Problem 3: Given the compound CaO, determine the sizes of its ions.
Steps to Solve:
1. Determine the ionic radii of its cation: Ca+2 has an ionic radius of 0.100 nm.
2. Determine the ionic radii of its anion: O-2 has an ionic radius of 0.140nm.
We can compare these values to those of another ionic compound as discussed. This provides insight into which exhibits larger lattice energy.
Summary Lattice Site Vs Interstitial Site
Lattice site and interstitial site are two different positions in a crystal lattice. The key difference between lattice site and interstitial site is that the lattice site is the position of constituent particles in the crystal lattice, whereas the interstitial site is a position between regular positions in the array of constituents of the crystal that can be occupied by other particles.
Reference:
1. Tan, T.y. âCompound Semiconductors: Diffusion.â Encyclopedia of Materials: Science and Technology, 2001, pp. 1425â1441., doi:10.1016/b0-08-043152-6/00263-1.
Image Courtesy:
1. âFace-centered cubic crystal lattice -â By Jcwf â Own work via Commons Wikimedia2. âSites interstitiels cubique a faces centreesâ By Christophe Dang Ngoc Chan â Own work via Commons Wikimedia
Also Check: Glencoe Geometry Chapter 7 Quiz 1 Answer Key
What Is Lattice Energy
Lattice energy is a measure of the strength of the ionic bonds in an ionic compound. It provides insight into several properties of ionic solids including their volatility, their solubility, and their hardness. The lattice energy of an ionic solid cannot be measured directly. However, it can be estimated with the help of the Born-Haber cycle. Generally, this quantity is expressed in terms of kilojoules per mole .
What Is Meant By The Term Lattice Chemistry
Chemistry Dictionary. A grid is an ordered grid of dots that describes the arrangement of the particles that make up a crystal. The elementary lattice of a crystal is determined by the points of the lattice. The cell is the smallest part of the crystal that, repeating regularly through three-dimensional translation, makes up the entire crystal.
Read Also: How To Figure Out Displacement
Introduction To Lattice Theory
Matter primarily can be classified as solid and fluid state. Any material whose position of constituent particles is fixed is called as solids. Solids are incompressible, rigid and possess mechanical strength which indicates the constituent molecules, atoms or ions that form the solid is closely packed. Thus, in solids, a well ordered molecular, atomic or ionic arrangement can be observed.
Solid matter can be classified into two types:
-
Crystalline solids .
-
Amorphous solids .
If the atoms or molecules are symmetrically arranged in crystalline solid or liquid it is called as crystal structure. A crystal possesses infinitely repeating order and symmetry. The entire crystal structure can be considered as the repetition of unit cell. For a particular crystal structure, the volume and shape of the unit cell is same but varies from crystal to crystal. The arrangement of constituent particles in a crystal in a definite pattern in the crystal can be observed by X-ray diffraction studies.