What Is Steric Effect With Example
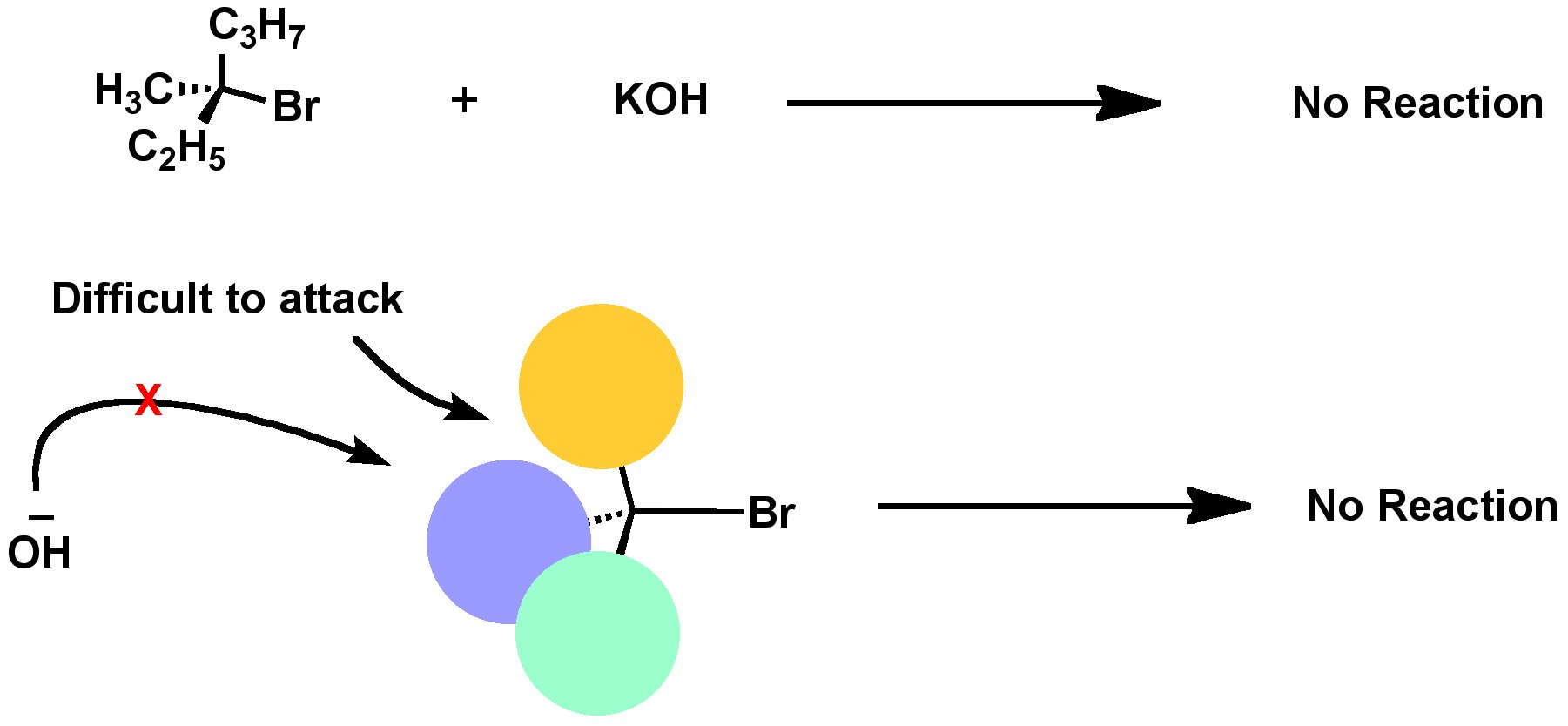
Steric effects are the effects seen in molecules that come from the fact that atoms occupy space. … An example of steric effects is steric hindrance. This is when a large group in a molecule makes reactions not work. For example, an SN2 reaction does not happen on carbon atoms that have three substituents.
What Is Meant By Steric Factor
factorsteric factordefineddefinedpre-exponential factor
. Also to know is, what is steric factor in organic chemistry?
Illustrated Glossary of Organic Chemistry – Steric effect. Steric effect: Any effect on a molecule, a reaction, etc. due to the size of atoms or groups. This is an example of a steric effect caused by steric hindrance: the tertiary carbon is more hindered than the primary carbon.
Beside above, what are steric requirements? A steric requirement is basically the energy it takes to overcome electrostatic repulsions from bringing atoms and molecules closer together. A proportionate amount of the kinetic energy from collisions is needed, thus slowing the actual rate of reaction .
In this way, what is meant by steric effect?
Steric effects are the effects seen in molecules that come from the fact that atoms occupy space. When atoms are put close to each other, this costs energy. The electrons near the atoms want to stay away from each other. This can change the way molecules want to react. An example of steric effects is steric hindrance.
What is orientation factor?
Explanation:Orientation factor is a number which lies between 0 and 1. It represents the fraction of collisions with an orientation which allows the reaction to take place.
How Do You Calculate Steric Factor
factorsteric factorfactor
Bobbi Solaz
steric hindrancesteric hindranceSteric hindrancechemical
Isidre Ahijado
inductive effecteffect
Bent De Puerta
organiceffecteffecteffectsorganic chemistry
Sevak Naia
Steric hindrancestericoccursSteric hindrance
Nit Nania
effectpositiveeffectnegative inductive effecteffect
Ramonita Latzuregui
You May Like: Formula For Volume Physics
Putting It All Together
The rate constant of the gas-phase reaction is proportional to the product of the collision frequency and the fraction of successful reactions. As stated above, sufficient kinetic energy is required for a successful reaction however, they must also collide properly . Compare the following equation to the Arrhenius equation:
where
- k is the rate constant for the reaction
- is the steric factor. It is the probability of the reactant molecules colliding with the right orientation and positioning to achieve a product with the desirable geometry and stereospecificity. The steric factor is very difficult to assess on paper it is determined experimentally.
- Zis the pre-exponential factor, A, of the Arrhenius equation. In theory, it is the frequency of total collisions that collide with the right orientation. In practice, it is the pre-exponential factor that is directly determined by experiment and then used to calculate the steric factor.
- Ea is activation energy
The collision frequency equation can thus be given as follows:
where:
- NA is number of molecules per unit volume
- kB is Boltzmann’s constant
Examples of gas-phase reactions and their steric factors
Reactions with more complex reactants and greater needs for collision orientation specificity, such as that below, have smaller steric factors .
The opposite holds true for simpler reactions they have a relatively larger steric factors:
Melting And Boiling Properties
Organic compounds typically melt and many boil. In contrast, while inorganic materials generally can be melted, many do not boil, and instead tend to degrade. In earlier times, the melting point and boiling point provided crucial information on the purity and identity of organic compounds. The melting and boiling points correlate with the polarity of the molecules and their molecular weight. Some organic compounds, especially symmetrical ones, sublime. A well-known example of a sublimable organic compound is para-dichlorobenzene, the odiferous constituent of modern mothballs. Organic compounds are usually not very stable at temperatures above 300 °C, although some exceptions exist.
Read Also: How To Calculate Displacement In Physics
Iii Results And Discussion
shows the results from the two energy decomposition analyses, Eqs. and , together with the steric energy difference result from the wavefunction theory for the 59 self-exchange SN2 reactions of substituted methyl halide systems, R1R2R3CX, with Fâ and R1, R2, and R3 = H, F, Cl, OH, CH3, CF3, OCH3, C2H5, C3H8, Ph, and para- and meta-substituted benzene groups. Gas-phase SN2 barrier height data from both computational and experimental studies have been reported elsewhere. ,,- For example, for CH3F , the experimental value of the barrier height in enthalpy difference is about 13.3 kcal/mol, our results are 9.22 kcal/mol in energy difference and 13.8 kcal/mol in enthalpy difference, indicating that our results are in general in reasonable agreement with the experimental data. Nevertheless, we notice that earlier studies have shown that to accurately reproduce the experimental data, high-level computational approaches at the G2, G3, or even higher level of theory are required.- Since the main purpose of this work is to qualitatively understand the nature of the barrier height in terms of energy decomposition schemes, the high accuracy and agreement with experimental data are not our major concern in this work.
Ii Methods And Computations
In DFT, the conventional energy difference ÎE between the transition state and reactants, is as follows-
where TS, Ee and Exc are the non-interacting kinetic, the electrostatic, and the exchange-correlation energy density functionals, respectively, and
with Vne, J, and Vnn defining the nuclear-electron attraction, classical electron-electron Coulomb repulsion, and nuclear-nuclear repulsion energies, respectively.
In the new energy decomposition scheme,, we assume that the energy difference comes solely from three independent effects, steric, electrostatic, and quantum ,
where the steric contribution is found to be the Weizsäcker kinetic energy
and the contribution from the quantum effect is the following
The main difference between Eqs. and is the identification of the Pauli component in the kinetic energy,- EPauliâ¡ TS – TW, which must be included as part of the contribution to the quantum effect.
The physical meaning of this description of steric effect in the framework of density functional theory is the introduction of a hypothetical reference state, where electrons are assumed to be Bosons. Physically, represents the minimal space of the hypothetical state occupied by the electrons when all of them are placed in the lowest orbital. This picture is in contrast with the wavefunction quantification of the steric effect where the Pauli Exclusion Principle was employed, which applies only to the same-spin electrons to account for their static correlation.
Also Check: Are Michael Jacksons Kids Biologically His
What Is Steric Factors In Organic Chemistry
Also called the probability factor, the steric factor is defined as the ratio between the experimental value of the rate constant and the one predicted by collision theory. It can also be defined as the ratio between the pre-exponential factor and the collision frequency, and it is most often less than unity.
What Is Steric Effect In Chemistry
In chemistry, a steric effect is an influence on a reaction’s course or rate determined by the fact that all of the atoms within a molecule occupy space, thus certain collision paths are either disfavored or favored. … Steric effects are frequently contrasted with electronic effects in explaining chemical reactivity.
Recommended Reading: Percent Error In Chemistry
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
What Is Electronic Effect Explain With An Example
The +E effect can be observed in the addition of acid to alkenes. … The +E effect is generally observed when the attacking reagent is an electrophile and the pi electrons are transferred towards the positively charged atom. An example where the +E effect occurs is the protonation of ethene which is illustrated below.
You May Like: Mcgraw Hill Geometry Workbook Answers
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.