Analysis Of Irreversible Inhibition
As shown in the figure to the right, irreversible inhibitors have a short instance where they form a reversible non-covalent complex with the enzyme and this then reacts to produce the covalently modified “dead-end complex” EI* . The rate at which EI* is formed is called the inactivation rate or kinact. Since formation of EI may compete with ES, binding of irreversible inhibitors can be prevented by competition either with substrate or with a second, reversible inhibitor. This protection effect is good evidence of a specific reaction of the irreversible inhibitor with the active site.
The binding and inactivation steps of this reaction are investigated by incubating the enzyme with inhibitor and assaying the amount of activity remaining over time. The activity will be decreased in a time-dependent manner, usually following exponential decay. Fitting these data to a rate equation gives the rate of inactivation at this concentration of inhibitor. This is done at several different concentrations of inhibitor. If a reversible EI complex is involved the inactivation rate will be saturable and fitting this curve will give kinact and Ki.
Inhibition Of A Catalyst
An inhibitor can reduce the effectiveness of a catalyst in a catalysed reaction . E.g., if a compound is so similar to the reactants that it can bind to the active site of a catalyst but does not undergo a catalytic reaction then that catalyst molecule cannot perform its job because the active site is occupied. When the inhibitor is released, the catalyst is again available for reaction.
Inhibition And Catalyst Poisoning
Inhibition should be distinguished from catalyst poisoning. An inhibitor only hinders the working of a catalyst without changing it, whilst in catalyst poisoning the catalyst undergoes a chemical reaction that is irreversible in the environment in question .
Index inhibitors or simplified as inhibitor predictably inhibit metabolism via a given pathway and are commonly used in prospective clinical drug-drug interaction studies.
Inhibitors of CYP can be classified by their potency, such as:
- Strong inhibitor being one that causes at least a 5-fold increase in the plasma AUC values, or more than 80% decrease in clearance of substrates .
- Moderate inhibitor being one that causes at least a 2-fold increase in the plasma AUC values, or 50-80% decrease in clearance of substrates .
- Weak inhibitor being one that causes at least a 1.25-fold but less than 2-fold increase in the plasma AUC values, or 20-50% decrease in clearance of substrates .
Read Also: What Is Automatic Processing In Psychology
Measuring The Dissociation Constants Of A Reversible Inhibitor
As noted above, an enzyme inhibitor is characterised by its two dissociation constants, Ki and Ki’, to the enzyme and to the enzyme-substrate complex, respectively. The enzyme-inhibitor constant Ki can be measured directly by various methods one extremely accurate method is isothermal titration calorimetry, in which the inhibitor is titrated into a solution of enzyme and the heat released or absorbed is measured. However, the other dissociation constant Ki’ is difficult to measure directly, since the enzyme-substrate complex is short-lived and undergoing a chemical reaction to form the product. Hence, Ki’ is usually measured indirectly, by observing the enzyme activity under various substrate and inhibitor concentrations, and fitting the data to a modified MichaelisMenten equation
- V
- . =1+^}}.}
Thus, in the presence of the inhibitor, the enzyme’s effective Km and Vmax become Km and Vmax, respectively. However, the modified Michaelis-Menten equation assumes that binding of the inhibitor to the enzyme has reached equilibrium, which may be a very slow process for inhibitors with sub-nanomolar dissociation constants. In these cases, it is usually more practical to treat the tight-binding inhibitor as an irreversible inhibitor however, it can still be possible to estimate Ki’ kinetically if Ki is measured independently.
What Are Corrosion Inhibitors
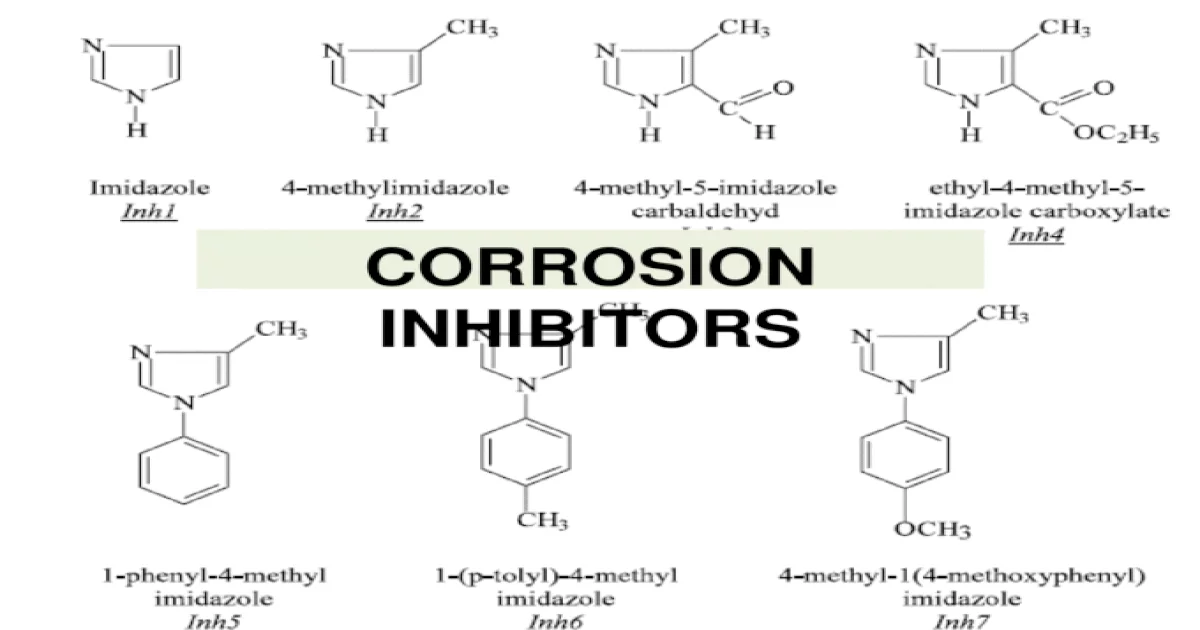
Corrosion Inhibitor is a chemical substance that is added to the environment in small concentrations to effectively reduce the rate of corrosion of the materials exposed in the same environment. Corrosion Inhibitor is usually denoted as CI.
- It acts as the first line defence against corrosion.
- These products tend to form a protective barrier on metal surfaces and break the chemical reaction, preventing rust to occur.
Also Read:
Recommended Reading: Geometry Dash World All Vault Codes
Examples Of Irreversible Inhibitors
Diisopropylfluorophosphate is shown as an example of an irreversible protease inhibitor in the figure above right. The enzyme hydrolyses the phosphorusfluorine bond, but the phosphate residue remains bound to the serine in the active site, deactivating it. Similarly, DFP also reacts with the active site of acetylcholine esterase in the synapses of neurons, and consequently is a potent neurotoxin, with a lethal dose of less than 100 mg.
Suicide inhibition is an unusual type of irreversible inhibition where the enzyme converts the inhibitor into a reactive form in its active site. An example is the inhibitor of polyamine biosynthesis, -difluoromethylornithine or DFMO, which is an analogue of the amino acid ornithine, and is used to treat African trypanosomiasis . Ornithine decarboxylase can catalyse the decarboxylation of DFMO instead of ornithine, as shown above. However, this decarboxylation reaction is followed by the elimination of a fluorine atom, which converts this catalytic intermediate into a conjugated imine, a highly electrophilic species. This reactive form of DFMO then reacts with either a cysteine or lysine residue in the active site to irreversibly inactivate the enzyme.
Suppression Of Gene Function
Many drugs act as suppressors of gene function including antibiotics, fungicides, antimalarials and antivirals. Gene function may be suppressed in several steps of protein synthesis or inhibition of nucleic acid biosynthesis. Many substances which inhibit nucleic acid biosynthesis are very toxic since the drug is not very selective in its action between the parasite and host.
You May Like: Algebra 1 Edgenuity Answers
Quantitative Description Of Reversible Inhibition
Reversible inhibition can be described quantitatively in terms of the inhibitor’s binding to the enzyme and to the enzyme-substrate complex, and its effects on the kinetic constants of the enzyme. In the classic Michaelis-Menten scheme below, an enzyme binds to its substrate to form the enzymesubstrate complex ES. Upon catalysis, this complex breaks down to release product P and free enzyme. The inhibitor can bind to either E or ES with the dissociation constantsKi or Ki’, respectively.
|
Kinetic scheme for reversible enzyme inhibitors |
Can Inhibitors Be Treated
Inhibitors are difficult and expensive to treat, and every case is different. Inhibitors can appear and disappear with treatment, and at times an inhibitor can disappear without treatment . Treatment options, such as high-dose clotting factor concentrates, bypassing agents, and immune tolerance induction therapy can help reduce the amount of inhibitors present.
Inhibitors require specialized medical expertise, and it is recommended that people with inhibitors seek treatment at an HTC because they specialize in treating people with bleeding disorders. Find an HTC.
Also Check: Books Never Written Pre Algebra With Pizzazz
Inhibitor Definition In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
In chemistry, an inhibitor is a substance that delays, slows or prevents a chemical reaction. It may also be called a negative catalyst.
Common Misspellings: inhibiter
There are three common classes of inhibitors:
- Corrosion inhibitor: A corrosion inhibitor decreases the rate of oxidation of metal.
- Enzyme inhibitor: In chemistry and biology, an enzyme inhibitor binds to an enzyme, lessening its activity. Enzyme inhibitors may be reversible or irreversible.
- Reaction inhibitor: A reaction inhibitor is any substance that decreases the rate of a chemical reaction. Corrosion inhibitors and enzyme inhibitors are both types of reaction inhibitors. Reaction inhibitors are classified by their potency as strong, moderate, or weak.
Applications Of Corrosion Inhibitors
Corrosion Inhibitors have a wide scope of applications in business, process, and industrial conditions. A few of these applications or their uses are as follows:
-
These Inhibitors are utilized to quit rusting and anodic corrosion of metals. This is commonly done through the coating of the metal surface with a chromate layer.
-
Oxygen scavengers can be utilized as CIs this reacts with the environment’s dissolved oxygen and can also help in the prevention of corrosion of the cathodic.
-
It is essential to prevent rusting and fuel pipeline corrosion. Hence, CIs are significant in making sure about these pipelines and diminishing the danger of mishaps.
-
Metal pipes used in the heating systems are inclined to corrosion. CIs play a significant role in making sure about these pipes also.
Also Check: What Type Of Math Do Architects Use
Discovery And Design Of Inhibitors
New drugs are the products of a long drug development process, the first step of which is often the discovery of a new enzyme inhibitor. In the past the only way to discover these new inhibitors was by trial and error: screening huge libraries of compounds against a target enzyme and hoping that some useful leads would emerge. This brute force approach is still successful and has even been extended by combinatorial chemistry approaches that quickly produce large numbers of novel compounds and high-throughput screening technology to rapidly screen these huge chemical libraries for useful inhibitors.
More recently, an alternative approach has been applied: rational drug design uses the three-dimensional structure of an enzyme’s active site to predict which molecules might be inhibitors. These predictions are then tested and one of these tested compounds may be a novel inhibitor. This new inhibitor is then used to try to obtain a structure of the enzyme in an inhibitor/enzyme complex to show how the molecule is binding to the active site, allowing changes to be made to the inhibitor to try to optimise binding. This test and improve cycle is then repeated until a sufficiently potent inhibitor is produced.Computer-based methods of predicting the affinity of an inhibitor for an enzyme are also being developed, such as molecular docking and molecular mechanics.
Examples Of Inhibitor In A Sentence
inhibitor New York Timesinhibitor ForbesinhibitorSELFinhibitor oregonliveinhibitorQuartzinhibitor NBC Newsinhibitor The Conversationinhibitor Forbes
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘inhibitor.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
You May Like: Geography Vs Topography
Types Of Reversible Inhibitors
Reversible inhibitors attach to enzymes with non-covalent interactions such as hydrogen bonds, hydrophobic interactions and ionic bonds. Multiple weak bonds between the inhibitor and the active site combine to produce strong and specific binding. In contrast to substrates and irreversible inhibitors, reversible inhibitors generally do not undergo chemical reactions when bound to the enzyme and can be easily removed by dilution or dialysis.
There are four kinds of reversible enzyme inhibitors. They are classified according to the effect of varying the concentration of the enzyme’s substrate on the inhibitor.
These types can also be distinguished by the effect of increasing the substrate concentration on the degree of inhibition caused by a given amount of inhibitor. For competitive inhibition the degree of inhibition is reduced by increasing , for noncompetitive inhibition the degree of inhibition is unchanged, and for uncompetitive inhibition the degree of inhibition increases with .
How Do I Know If I Have An Inhibitor
Only a blood test can determine if an inhibitor is present and it can measure the amount present. Inhibitors are complex, and not everyone with hemophilia and VWD type 3 will develop an inhibitor. Researchers do not yet know why some people will develop an inhibitor and why some will not, but research studies are being conducted to learn more about them. Inhibitors can appear at any time, so it is important that all people with hemophilia and VWD type 3 be tested for an inhibitor each year.
Eligible individuals can receive free inhibitor testing at federally funded hemophilia treatment centers through the Community Counts Registry for Bleeding Disorders Surveillance.
Read Also: Define Elastic Force
What Is A Corrosion Inhibitor
A corrosion inhibitor is a chemical solvent that is applied in a given environment to decrease the pace of corrosion of the metal which is exposed to that particular condition, for example, air or water. CI can be the abbreviation of the Corrosion Inhibitor.
A corrosion Inhibitor can be defined as a chemical compound that can be added to fluids or gases and used to diminish the corrosion pace of a given material . One of the techniques for the Corrosion Inhibitor can be the addition of a coating on the metals surface which goes about as a passivation layer and denies access to the metal surface.
Important Compounds And Applications
10.16.7.10.1Pyridopyridazines
A new class of enzyme inhibitors, such as pyridopyridazine 1021 and 1022, have IC50 of 0.2 and 0.6 against p38 map kinase, respectively.91
10.16.7.10.2Pyrido/pyrano/thiopyrano pyrimidines
Compounds containing pyridopyrimidine and pyridopyrimidin-7-ones display a wide range of medicinal and pharmacological activities, including inhibition of p21-activated kinases,477 mitogen-activated protein kinase 4,478 rapidly accelerated fibrosarcoma kinases,192 Wee-1 kinases,479 and hepatitis C virus,480 along with specific treatment of lymphoma by 1023.481 Studies have identified 1024 as a low-nanomolar cell-based dual Akt 1 and 2 inhibitors.482 Interestingly this ring system, as observed in 1025, was identified as a new class of fibroblast growth factor receptor tyrosine kinase inhibitor.483 Pyridopyrimidine 1026 selectively inhibits dihydrofolate reductase with an IC50 of 0.0042 mM for Pneumocystis jirovecii DFHR,484 a causative agent of pneumonia in HIV/AIDS patients.
10.16.7.10.3Pyrido pyrazines
A range of biological activity has been observed for compounds containing pyridopyrazines derivatives, including extracellular signalregulated kinase 2 inhibition by 1041,505 p38R MAP kinase inhibition by 1042 ,506 and transglutaminase 2 inhibition by 1043.507
10.16.7.10.4Pyrido oxazines
Cecilia Pozzi, … Stefano Mangani, in, 2018
You May Like: Is Physics Harder Than Chemistry
Where Can I Learn More
Inhibitors can be challenging, but there are organizations, support communities, and resources available to help support people with bleeding disorders and inhibitors.
- Visit the CDCs website on inhibitors, and download free fact sheets on inhibitors.
- Learn about Community Counts and how you can help advance research on bleeding disorders and inhibitors.
- Seek support and resources from the National Hemophilia Foundationexternal icon and the Hemophilia Federation of Americaexternal icon.
Corrosion Inhibitor: Definition Types Application And Mechanisms
Content Curator
Our nature is the biggest example of how matter exhibits change from time to time. Some of the changes that we observe in our everyday lives may include fruits getting rotten, milk turning into curd, iron nails and other substances turning reddish brown with passing time, precious metals like gold silver losing their lustre and other innumerable changes. These changes are called Chemical Changes. One such chemical reaction where the metal reacts with the surrounding to change their state is called corrosion.
|
Table of Content |
Read More: Nitrogen Dioxide
You May Like: Cyte Root Word
Different Kinds Of Corrosion Inhibitors
The substance which is used to inhibit the corrosion rate are called corrosion inhibitors. Fundamentally there are three types of corrosion inhibitors namely Anodic inhibitors, Cathodic inhibitors, and Mixed inhibitors.
- Anodic Inhibitors:Anodic inhibitors also known as passivators are chemical substances that form a protective layer of oxide on the metal surface resulting in resistance to corrosion. These inhibitors alter the anodic reactions in a chemical cell, forcing the metal into passivate region. Anodic inhibitors are mostly considered unsafe due to their chemical properties.
Examples of anodic inhibitors might include:
- Cathodic Inhibitors:Cathodic inhibitors either slow down the cathodic reaction itself or selectively precipitate on cathodic areas to increase the surface resistivity and limit the distribution of reducible species to these areas.
There are three different mechanisms by which cathodic inhibitors can provide inhibition:
Examples Of Reversible Inhibitors
As enzymes have evolved to bind their substrates tightly, and most reversible inhibitors bind in the active site of enzymes, it is unsurprising that some of these inhibitors are strikingly similar in structure to the substrates of their targets. Inhibitors of DHFR are prominent examples. Other example of these substrate mimics are the protease inhibitors, a very successful class of antiretroviral drugs used to treat HIV. The structure of ritonavir, a protease inhibitor based on a peptide and containing three peptide bonds, is shown on the right. As this drug resembles the protein that is the substrate of the HIV protease, it competes with this substrate in the enzyme’s active site.
Enzyme inhibitors are often designed to mimic the transition state or intermediate of an enzyme-catalyzed reaction. This ensures that the inhibitor exploits the transition state stabilising effect of the enzyme, resulting in a better binding affinity than substrate-based designs. An example of such a transition state inhibitor is the antiviral drug oseltamivir this drug mimics the planar nature of the ring oxonium ion in the reaction of the viral enzyme neuraminidase.
Read Also: Beth Child Of Rage Where Is She Now