What Is The Composition Of Matter In Chemistry
Matterchemical composition
. Simply so, what is matter in general chemistry?
Chemists study the structures, physical properties, and chemical properties of material substances. These consist of matter, which is anything that occupies space and has mass. The mass of an object is the quantity of matter it contains.
Subsequently, question is, is toothpaste homogeneous or heterogeneous? Can be in solid, liquid, or gas form! Milk, toothpaste, and mayonnaise are homogeneous mixtures. Heterogeneous mixtures You can see the particles that make it up.
Besides, what is mixture in chemistry?
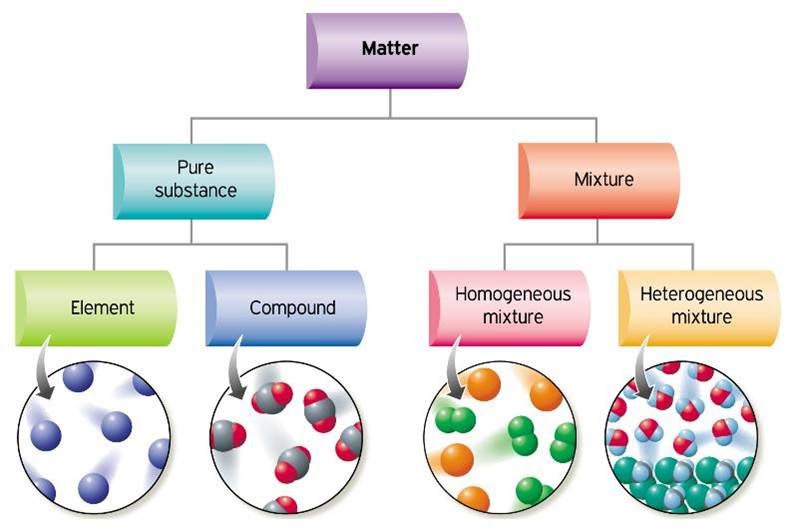
In chemistry, a mixture is a material made up of two or more different substances which are physically combined. A mixture is the physical combination of two or more substances in which the identities are retained and are mixed in the form of solutions, suspensions and colloids.
What does a fixed composition mean?
Every samples of a given substance has the same properties because the substance has a fixed, uniform composition. An element has a fixed composition because it contains only one type of atom. A compound always contains two or more elements in a fixed proportion.
In General Relativity And Cosmology
In the context of relativity, mass is not an additive quantity, in the sense that one can not add the rest masses of particles in a system to get the total rest mass of the system.:21 Thus, in relativity usually a more general view is that it is not the sum of rest masses, but the energymomentum tensor that quantifies the amount of matter. This tensor gives the rest mass for the entire system. “Matter” therefore is sometimes considered as anything that contributes to the energymomentum of a system, that is, anything that is not purely gravity. This view is commonly held in fields that deal with general relativity such as cosmology. In this view, light and other massless particles and fields are all part of “matter”.
Binding Forces And Massive Quarks
The binding forces carried by the gluons tend to be weak when quarks are close together. Within a proton , at distances of less than 1015 metre, quarks behave as though they were nearly free. This condition is called asymptotic freedom. When one begins to draw the quarks apart, however, as when attempting to knock them out of a proton, the effect of the force grows stronger. This is because, as explained by QCD, gluons have the ability to create other gluons as they move between quarks. Thus, if a quark starts to speed away from its companions after being struck by an accelerated particle, the gluons utilize energy that they draw from the quarks motion to produce more gluons. The larger the number of gluons exchanged among quarks, the stronger the effective binding forces become. Supplying additional energy to extract the quark only results in the conversion of that energy into new quarks and antiquarks with which the first quark combines. This phenomenon is observed at high-energy particle accelerators in the production of jets of new particles that can be associated with a single quark.
Read Also: What Does Reduce Mean In Math
Physical Properties Of Matter
Physical properties are properties that can be measured or observed without changing the chemical nature of the substance.
Some examples of physical properties are:
- Color .
- Boiling point : the temperature at which a substance boils.
- Melting point : the temperature at which a substance melts.
Physical properties Matter has mass and volume, as demonstrated by this concrete block. You can observe its mass by feeling how heavy it is when you try to pick it up you can observe its volume by looking at it and noticing its size.
Mass and volume are both examples of extensive physical properties.
Extensive Property
Any characteristic of matter that depends on the amount of matter being measured.
Intensive Property
Any characteristic of matter that does not depend on the amount of the substance present.
Changing States Of Matter
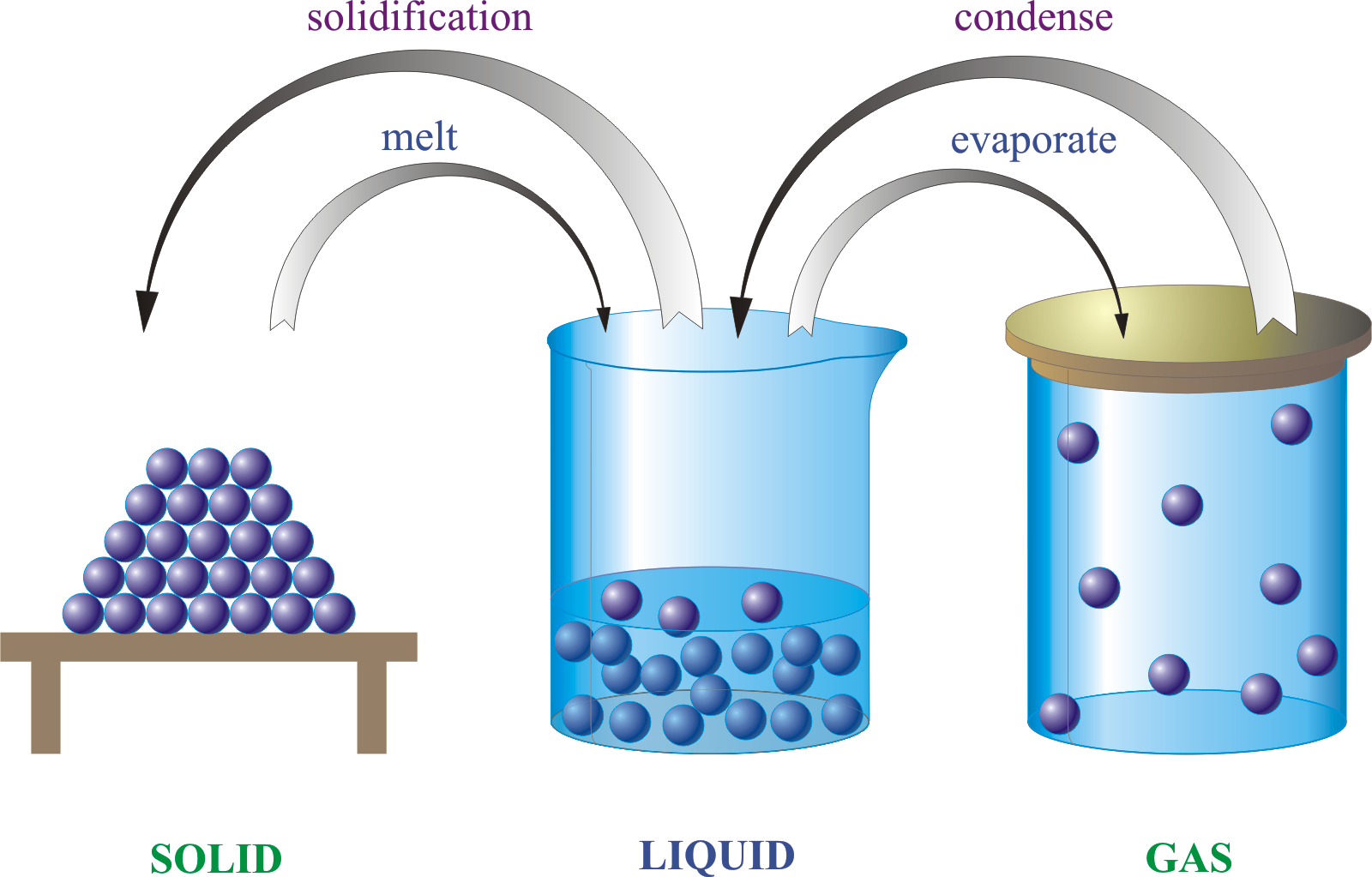
Molecules can move from one physical state to another and not change their atomic structure.
Oxygen gas has the same chemical properties as liquid oxygen. The liquid state is colder and denser , but the molecules are the same. Water is another example. A water molecule is made up of two hydrogen atoms and one oxygen atom.
It has the same molecular structure whether it is a gas, liquid, or solid. Although its physical state may change because of different amounts of energy, its atomic structure remains the same. So what is a chemical change in matter?
Lets start with that glass of pure water. If the formula of water were to change, that would be achemical change. If you could add a second oxygen atom to a water molecule, you would have hydrogen peroxide .
The molecules would not be water anymore. In reality, there are a variety of steps that go into creating hydrogen peroxide from water. Physical changes are related to changes in the immediate environment such as temperature, pressure, and other physical forces.
Chemical changes occur when the bonds between atoms in a compound are created or destroyed. Generally, the basic chemical structure does not change when there is a physical change. Of course, in extreme environments such as the Sun, no molecule is safe from destruction.
You May Like: Algebra 1 Eoc Practice Packet
Section 1: Further Reading
Pawley, Emily. Powerful Effervescence. Chemical Heritage Magazine 26 . http://www.chemheritage.org/discover/media/magazine/articles/26-2-powerful-effervescence.aspx.
Boyd, Jane E. Artificial Clouds and Inflammable Air: The Science and Spectacle of the First Balloon Flights, 1783. Chemical Heritage Magazine 27 . http://www.chemheritage.org/discover/media/magazine/articles/27-2-artificial-clouds-and-inflammable-air.aspx.
National Science Resource Center. The Water Cycle: From the Sky to the Land and Back Again. Properties of Matter, Lesson 7. http://www.propertiesofmatter.si.edu/Water_Cycle.html.
Schachtman, Tom. Absolute Zero and the Conquest of Cold. New York: Houghton Mifflin, 1999.
Water In Three States
Water can exist in three different physical statesas a gas, liquid, and a solidunder natural conditions on Earth. Regardless of its physical state, they all have the same chemical composition. Water is composed of two hydrogen atoms bonded to an oxygen atom.
2H2 + O2 -> 2H2O
Next, consider the plants and algae living in and along the stream. In a process called photosynthesis, these organisms convert light energy from the sun into chemical energy stored in sugars. However, the light energy doesn’t produce the atoms that make up those sugarsthat would break the Law of Conservation of Mass. It simply provides energy for a chemical change to occur. The atoms come from carbon dioxide in the air and water in the soil. Light energy allows these bonds to break and reform to produce sugar and oxygen. This is shown in the chemical equation for photosynthesis:
6CO2 + 6H2O + light -> C6H12O6 + 6O2
Water can exist in three different physical statesas a gas, liquid, and a solidunder natural conditions on Earth. Regardless of its physical state, they all have the same chemical composition. Water is composed of two hydrogen atoms bonded to an oxygen atom.
atom
the basic unit of an element, composed of three major parts: electrons, protons, and neutrons.
diatomic
Recommended Reading: Theory Of Everything Geometry Dash 2
Based On Quarks And Leptons
Under the quarks and leptons definition, the elementary and composite particles made of the quarks and leptons would be matterwhile the gauge bosons would not be matter. However, interaction energy inherent to composite particles contribute to the mass of ordinary matter.
As seen in the above discussion, many early definitions of what can be called ordinary matter were based upon its structure or building blocks. On the scale of elementary particles, a definition that follows this tradition can be stated as: ordinary matter is everything that is composed of quarks and leptons, or ordinarymatter is everything that is composed of any elementary fermions except antiquarks and antileptons.
The connection between these formulations follows. Leptons , and quarks combine to form atoms, which in turn formmolecules.
Because atoms and molecules are said to be matter, it is natural to phrase the definition as: ordinary matter is anything that is made of the same things that atoms and molecules are made of.
Then, because electrons are leptons, and protons, and neutrons are made of quarks, this definition in turn leads to the definition of matter as being quarks and leptons, which are two of the four types of elementary fermions .
Carithers and Grannis state: Ordinary matter is composed entirely of first-generation particles, namely the and quarks, plus the electron and its neutrino.
What Is Matter In Chemistry
As discovered by scientists,
the matter is made up of very tiny particles and these particles are so small that we cannot see them with naked eyes.
It has been observed that matter exists in nature in different forms. Some substances are rigid and have a fixed shape like wood and stone some substances can flow and take the shape of their container like water, while there are forms of matter that do not have definite shape or size such as air.
Read Also: Find Average Speed Physics
Chemistry Is A Physical Science
Chemistry is typically considered a physical science, as defined by the Encyclopedia Britannica, because the study of chemistry does not involve living things. Most of the chemistry involved in research and development, such as making new products and materials for customers, falls within this purview.
But the distinction as a physical science becomes a bit blurry in the case of biochemistry, which explores the chemistry of living things, according to the Biochemical Society. The chemicals and chemical processes studied by biochemists are not technically considered “living,” but understanding them is important to understanding how life works.
Section : Picturing Material Behavior With Phase Diagrams
Once the gas laws were formulated, chemists could analyze how materials transitioned from one phase to another, and how temperature and pressure affected these changes. In 1897, a British metallurgist named Sir William Chandler Roberts-Austen produced what is widely regarded as an early form of a now-common tool in chemistry and related disciplines: the phase diagram. Roberts-Austen and other English scientists were analyzing the properties of wootz steel, a metal that had been produced in India for centuries and was prized for making extremely sharp and durable weapons.2 His diagram showed how the composition of iron changed as it was heated.
Modern phase diagrams show relationships between different states of matter under various combinations of temperature and pressure. As we saw in Section 2, a substance can exist in two different states at oncefor example, as a liquid and a gas, with molecules cycling from one state to the other. It is also possible for a material to be both solid and liquid, with both melting and freezing taking place at its edges, or to exist as a solid and a gas. Phase diagrams show what forms a substance will take under given temperatures and pressure levels, and where these equilibrium lines are located.
Important parts of the phase diagram in Figure 2-11 include:
Don’t Miss: What Is Geology In Geography
Section : Measuring Temperature
Before chemists could analyze phase changes, they had to be able to measure temperature accurately. Although early scientists did not realize it, temperature is a measure of the average kinetic energy of the molecules in a substance, so it is directly related to states of matter.
In 1597, Italian physicist Galileo Galilei demonstrated the idea that water in a tube could be used to measure temperature because the water expanded when heated and contracted when cooled. However, the device Galileo created was a thermoscope, not a thermometer: It did not have a temperature scale, so it simply registered that the amount of heat was changing. Over the next century, other European scientists built enclosed glass thermometers that contained alcohol or oil to measure temperature, but none were widely adopted. Moreover, without a fixed temperature scale, no two scientists measurements were directly comparable.
Swedish astronomer Anders Celsius created another new scale in 1742 that set the freezing point of water at 0° and the boiling point at 100°. He called the scale Centigrade, reflecting that it was based on a 1-to-100 scale the system was renamed Celsius in his honor after his death. The Fahrenheit and Celsius scales are equal at -40° but diverge from that point, since each one-degree increase Celsius represents a much larger increment of temperature than one degree Fahrenheit.
Phases And Classification Of Matter
- Describe the basic properties of each physical state of matter: solid, liquid, and gas
- Distinguish between mass and weight
- Apply the law of conservation of matter
- Classify matter as an element, compound, homogeneous mixture, or heterogeneous mixture with regard to its physical state and composition
- Define and give examples of atoms and molecules
Matter is defined as anything that occupies space and has mass, and it is all around us. Solids and liquids are more obviously matter: We can see that they take up space, and their weight tells us that they have mass. Gases are also matter if gases did not take up space, a balloon would not inflate when filled with gas.
Solids, liquids, and gases are the three states of matter commonly found on earth . A solid is rigid and possesses a definite shape. A liquid flows and takes the shape of its container, except that it forms a flat or slightly curved upper surface when acted upon by gravity. Both liquid and solid samples have volumes that are very nearly independent of pressure. A gas takes both the shape and volume of its container.
Recommended Reading: Exponential Growth And Decay Worksheet Algebra 2 Answers
Solids Liquids And Gas
In asolid, particles are packed tightly together so they don’t move much. The electrons of each atom are constantly in motion, so the atoms have a small vibration, but they are fixed in their position. Because of this, particles in a solid have very low kinetic energy.
Solids have a definite shape, as well as mass and volume, and do not conform to the shape of the container in which they are placed. Solids also have a high density, meaning that the particles are tightly packed together.
In aliquid, the particles are more loosely packed than in a solid and are able to flow around each other, giving the liquid an indefinite shape. Therefore, the liquid will conform to the shape of its container.
Much like solids, liquids are incredibly difficult to compress.
In agas, the particles have a great deal of space between them and have high kinetic energy. A gas has no definite shape or volume. If unconfined, the particles of a gas will spread out indefinitely if confined, the gas will expand to fill its container. When a gas is put under pressure by reducing the volume of the container, the space between particles is reduced and the gas is compressed.
Show A Video So That Students Can See An Example That Water Molecules Are Attracted To One Another
Ask students:
Students should realize that water holds together pretty well because the water molecules are attracted to each other.
Tell students that they should answer these questions on their activity sheet.
Extra Extend
Also Check: Holt Pre Algebra Homework And Practice Workbook Answer Key
Chemistry Glossary Definition Of State Of Matter
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Physics and chemistry both study matter, energy, and interactions between them. From the laws of thermodynamics, scientists know matter can change states and the sum of the matter and energy of a system is constant. When energy is added or removed to matter, it changes state to form a state of matter. A state of matter is defined as one of the ways in which matter can interact with itself to form a homogeneous phase.
Section : Measuring Pressure
Pressure is a simple concept: It measures the amount of force that is pushing in a given direction on a defined area . However, scientists have developed many different methods and units for measuring pressure. The unit that is most widely recognized internationally for measuring pressure is the pascal , a metric unit named after French mathematician and philosopher Blaise Pascal . One pascal is equal to one newton per square meter, which is a very small unit. As discussed above, standard air pressure is 14.7 pounds per square inch, which is equivalent to 101,325 pascals. Because this number is so large and difficult to work with, measurements are often listed in hectopascals or kilopascals for ease of use.
Other common units of pressure include:
Torricelli and the Invention of the Barometer
Italian physicist and mathematician, Evangelista Torricelli first tried constructing a barometer that used water to measure pressure. He filled a 60-foot-long glass tube with a closed bulb at one end with water and inverted the tube into a basin filled with more water. The top of the water column fell slightly, but it did not drain completely and flow over the sides of the basin. This showed, in Torricellis view, that the atmospheric pressure was pushing down on the open surface of the water in the basin, which forced the water up in the tube.
Recommended Reading: Evaluating Functions Worksheet Algebra 2 Answer Key