Equivalent Weights Of Salts
To calculate the equivalent weight of a compound that is neither an acid nor a base, we need to know the charge of the cation or anion. The mass of the cation divided by the charge on it is called the equivalent mass of the cation, and the mass of the anion divided by the charge on it is called the equivalent mass of the anion. When we add equal masses of anions and cations, it gives us equal amounts of salt. For salts, the valence factor is the total amount of positive or negative charges provided by the 1 mol of salt.
Classification Of Inorganic Compounds
The Inorganic compounds that are classified as:
- Acids Acids are those compounds that dissolve in water and generate hydrogen ions or H+ Ions. The examples of acids include Hydrochloric acid, citric acid, sulphuric acid, vinegar, etc. One example of the acidic reaction is shown below-Hydrochloric acid + water H+ + Cl
- Bases A base is a type of substance or a compound that produces hydroxyl ions when kept in water. The bases like potassium hydroxide, calcium hydroxide, ammonia, sodium hydroxide produce OH- ions when dissolved in water. Potassium Hydroxide + H2O K+ + OH
- Salts As you might be familiar with the word Salt. The substances obtained as a result of the reaction between an acid and a base are called Salts. The table salt of sodium hydroxide is one of the typical examples of salts.
- Oxides The compounds which consist of one oxygen atom called Oxides.
What Is An Electrochemical Cell
An electrochemical cell is a piece of equipment that can create an electrical form of energy via chemical reactions. This produces energy to assist in various chemical reactions.
Class 12 Chemistry Electrochemistry notes further explain that this form of a cell can alter chemical energy into electrical. Moreover, electrical power to transform into chemical energy as per chapter 3 class 12 Chemistry notes is explained. The typical example of this form of a cell is the 1.5-volt battery used in day-to-day life. These batteries used to power tv remotes, clocks, toys, etc.
The best part of these cells is that they can generate current from reactions like a galvanic cell. Moreover, they can lead to chemical reactions when an electric current passes through. This becomes the base of electrolytic cells in class 12 Chemistry chapter 3 Electrochemistry notes.
This brings us to question what a Galvanic cell is? This has been explained in a detailed manner in class 12, chapter 3 Chemistry notes. Take a look!
Don’t Miss: Reversible Figure-ground Relationship
Reactions In Aqueous Media
The two solids cannot react with each other in the solid phase, so they must be dissolved in the liquid. When solutes dissolve in a solvent, they coexist in a single phase called a solution. Several parameters are used to measure the strength of the solution. The strength of a solution denotes the amount of solute which is contained in the solution.
The parameters used to denote the strength of a solution are:
-
Mole dfraction \: moles of a component / Total moles of solution.
-
Mass\ : Mass of solute present in \ of solution.
-
\ : Mass of solute present in \ of solution
-
\ : Volume of solute/volume of solution
-
\ : Wt. of solute in \ of solution
-
ppm : \
-
Molarity :\
-
Molality :\
Important Relations
1. Relation between molality Molarity , density of solution and molar mass of solute
\ : density in g/mL
\: molar mass in \
Molality, \
2. Relationship between molality and mole dfraction of the solute
$m=\dfrac_}}_}}\times \dfrac_}}$
$m=\dfrac_}}_}}\times \dfrac_}}$
Points to remember:
-
Molarity is the most common unit of measuring strength of solution.
-
The product of Molarity and Volume of the solution gives the number of moles of the solute, \
-
All the formulae of strength have an amount of solute. in the numerator.
-
All the formulae have an amount of solution in the denominator except for molality .
Classification Of Matter At Macroscopic Level
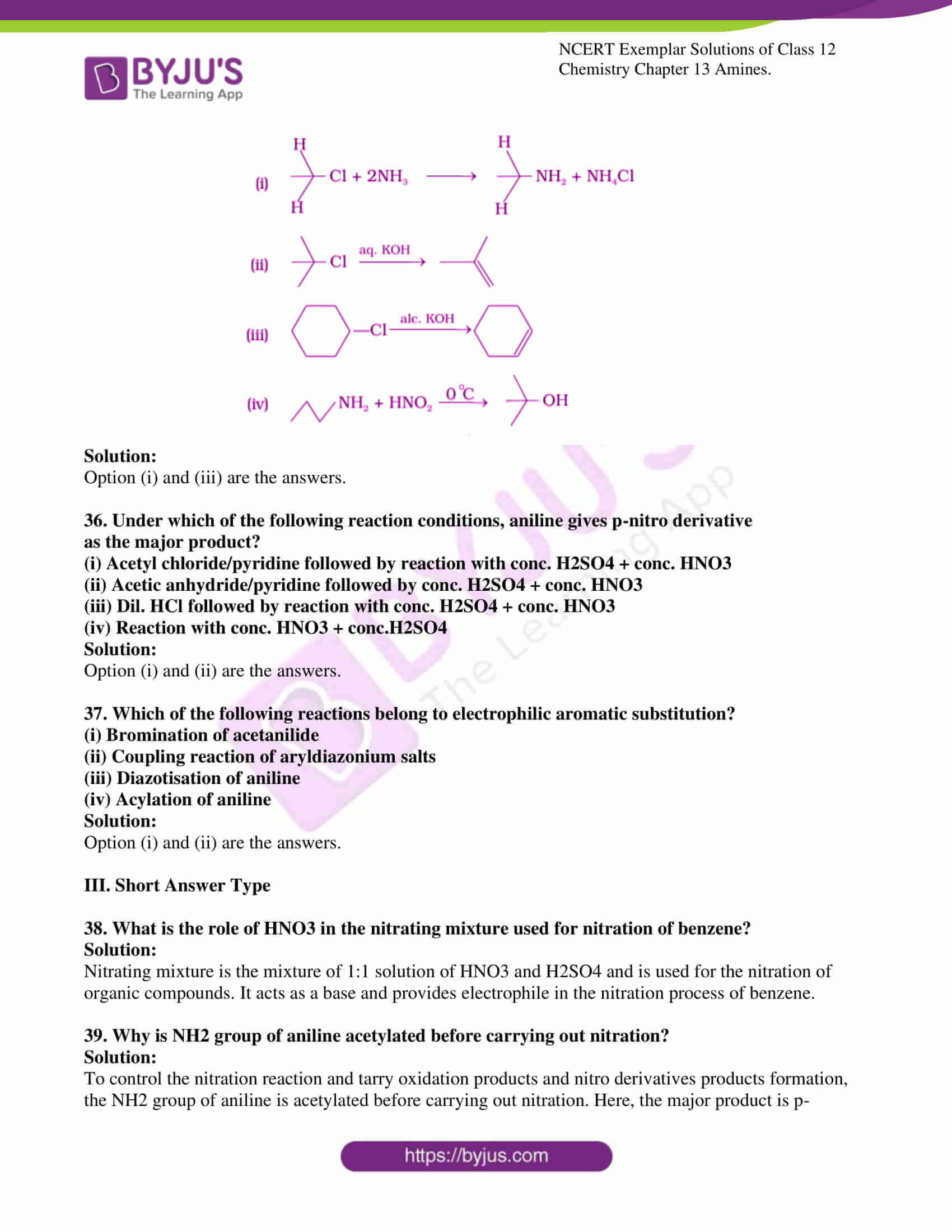
Matter can further be classified into following at bulk or macroscopic level:
Mixtures Pure Substances.
These can be further classified as shown below:
Mixtures : A mixture is a substance in which two or more substances are present in any ratio. Primarily, It is of two types: Heterogeneous and Homogeneous mixtures.
-
Homogeneous Mixture- Two substances are mixed to form a mixture such that there exist one single uniform phase i.e. composition of substances present is uniform. Sugar solution and air are thus, the examples of homogeneous mixtures.
-
Heterogeneous Mixtures- Two or more substances are mixed which result in non-uniform composition throughout the mixture. Some of the examples are suspensions, a mixture of two solids, suppose salt and sugar.
Note: Any distinct portion of matter that is uniform throughout in composition and properties is called a Phase.
Pure Substances:- A material containing only one type of particle is called a pure substance.
Note: In chemistry, forms of matter have constant chemical composition and chemical properties and they cannot be separated into components by physical methods.
Pure substances are further divided as given below:
Don’t Miss: What Is Ap Biology In High School
What Is The Meaning Of Ch Abbreviation In Organic Chemistry
What is CH definition ?
CH definition is “Cuticular Hydrocarbon”.
What does CH mean in Organic Chemistry?
CH mean that “contact hypersensitivity” for Organic Chemistry.
What is CH acronym ?
CH acronym is “Cytogenetic Heteromorphism”.
What is shorthand of Compounds Chloral Hydrate ?
The shorthand of “Compounds Chloral Hydrate” is CH.
What is the definition of CH acronym in Organic Chemistry?
Definitions of CH shorthand is “Calponin homology”.
What is the full form of CH abbreviation?
Full form of CH abbreviation is “Compounds Chloral Hydrate”.
What is the full meaning of CH in Organic Chemistry?
Full meaning of CH is “Cytogenetic Heteromorphism”.
What is the explanation for CH in Organic Chemistry?
Explanation for CH is “Calponin homology”.
What is the meaning of CH Abbreviation in Astrology ?
The site does not only include the meanings of the CH abbreviation in Organic Chemistry. Yes, we know your main purpose is explanation of CH abbreviation in Organic Chemistry. However, we thought that besides the meaning of the CH definitions in Organic Chemistry, you can consider astrological information of CH acronym in Astrology. Therefore, the astrological explanation of each word in each CH abbreviation is also included.
CH Abbreviation in Astrology
Notes For Chapter 1 Class 11 Chemistry Some Basic Concepts Of Chemistry
Class 11 Chapter 1 Some Basic Concepts of Chemistry is an introductory chapter but very crucial to understand for students as it forms the basis of Chemical reactions happens around us. To understand these concepts, Class 11 Chapter 1 Chemistry Notes are prepared by subject experts in well-defined and easy language. The goal of notes of Chemistry Class 11, Chapter 1 is to study just one topic and make short, readable notes to help you quickly remember the core points before the test. This is how you won’t feel like panicking before the exam.
Class 11th Chemistry Chapter 1 Notes are available in PDF file on the official website of Vedantu and its app to download for free by the students. Students can use Class 11 Chemistry ch 1 notes in PDF file both offline and online to understand the critical terms and concepts in easy to understand language with examples, diagrammatic explanations and solved problems.
Study without Internet
Don’t Miss: Molecular Shape Of Ccl4
What Do Chemists Do
Chemists work in a variety of fields, including research and development, quality control, manufacturing, environmental protection, consulting and law. They can work at universities, for the government or in private industry, according to the ACS.
Here are some examples of what chemists do:
Research and development
In academia, chemists performing research aim to further knowledge about a particular topic, and may not necessarily have a specific application in mind. Their results, however, can still be applied to relevant products and applications.
In industry, chemists in research and development use scientific knowledge to develop or improve a specific product or process. For example, food chemists improve the quality, safety, storage and taste of food pharmaceutical chemists develop and analyze the quality of drugs and other medical formulations and agricultural chemists develop fertilizers, insecticides and herbicides necessary for large-scale crop production.
Sometimes, research and development may not involve bettering the product itself, but rather the manufacturing process involved in making that product. Chemical engineers and process engineers devise new ways to make the manufacturing of their products easier and more cost effective, such as increasing the speed and/or yield of a product for a given budget.
Environmental protection
Additional resources:
How Do You Make Na2co3 Solution
Weigh the calculated amount of sodium carbonate on the scale. Pour distilled water20 to 30 mL less than the final volumeinto the beaker, then add sodium carbonate. In our example, start with 270 to 280 mL of water. Mix the solution with a spoon or gently swirl the beaker until the salt dissolves completely.
You May Like: Ksp Chemistry
Introduction To Equivalent Concept
The concept of equivalence is a way to understand chemical reactions and processes, and the concept of equivalence is usually used for simplification.
17.1 Equivalent Mass
The mass of an acid which furnishes \ mol \ is called its Equivalent mass.
The mass of the base which furnishes \ mol \ is called its Equivalent mass.
17.2 Valence Factor
Valence factor: is the number of \ ions supplied by \ molecule or mole of an acid or the number of \ ions supplied by \ molecule or \ mole of the base.
Mass Equivalent,$E=\dfrac}}$
17.3 Equivalents
No. of equivalents \
Note: It should be always remembered that \ equivalent of an acid reacts with \ equivalent of a base.
What Is Chapter 2 Of Ncert Class 12
All About The Chapter 2 Chemistry Class 12 Solution Chapter 2 of NCERT Chemistry is based on the basic idea of Solutions and its types. This chapter covers Concentration of Solutions, Solubility, Vapour Pressure of Liquid Solutions, Ideal and Non-Ideal Solutions, Colligative Properties and Determination and Abnormal Molar Masses.
Also Check: Holt Mcdougal Geometry Workbook Answers
Origin Of Equivalent Concept
The equivalent weight of an element was originally defined as the weight of the element combined with 1 grams of hydrogen. Subsequently, the definition was modified as follows: the equivalent weight of an element is the weight of the element combined with 8 grams of oxygen. Note: Same element can have multiple equivalent weights depending upon the charge on it. e.g. \ and \
Classification Of Carbon Atoms
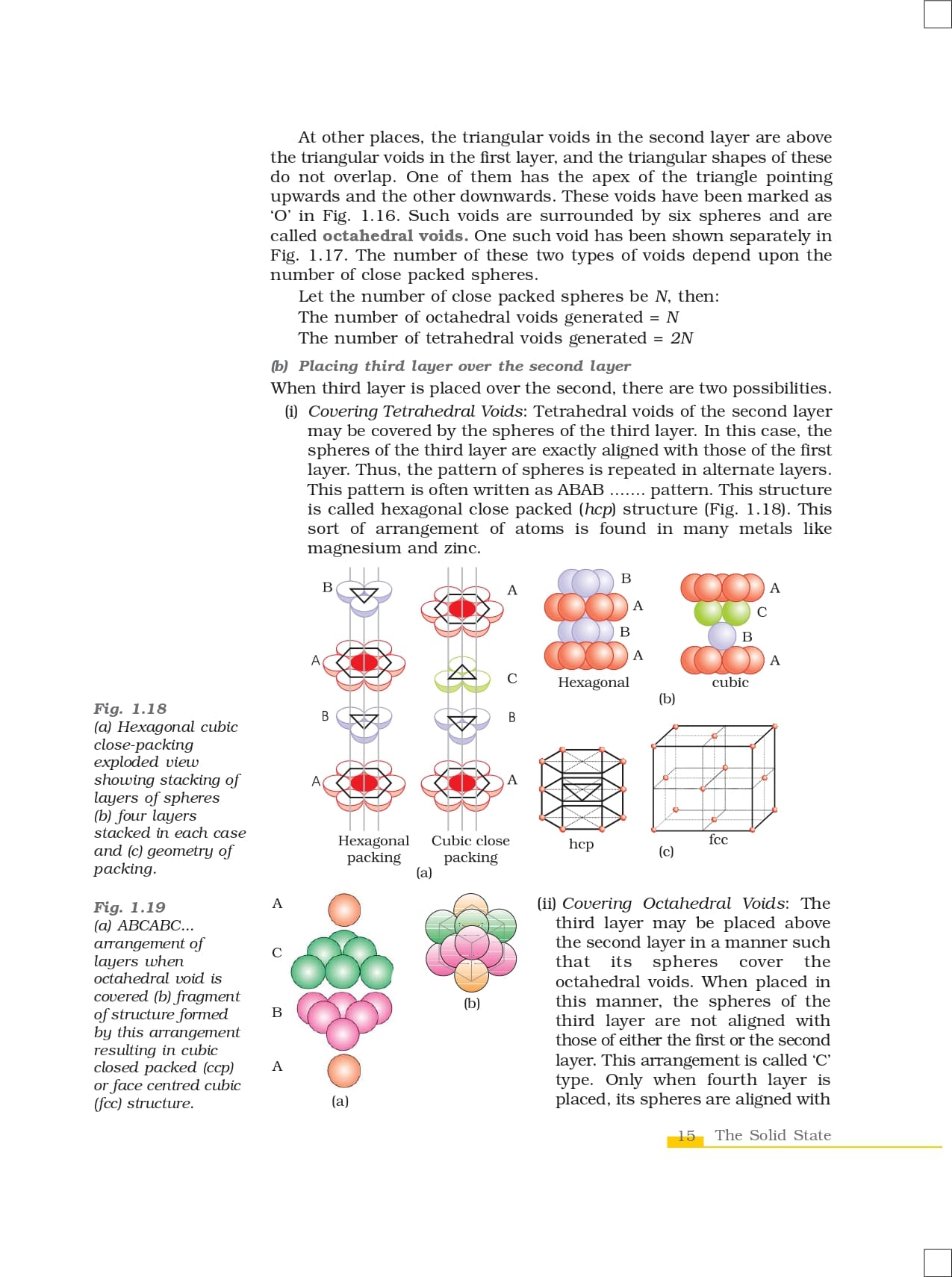
Carbons have a special terminology to describe how many other carbons they are attached to. This allows for an easy description of branching in alkanes. Also, we will find that the number of carbons attached to a given atom will have subtle effects on its chemistry.
- Primary carbons attached to one other C atom.
- Secondary carbons are attached to two other Cs.
- Tertiary carbons are attached to three other Cs.
- Quaternary carbons are attached to four C’s.
The figure below use the group “R” to represent an alkyl group of unspecified length. R typically used to represent alkyl groups but an also represent a part of a molecule which is either unspecified or not germane to the discussion.
This terminology will be used repeatedly in organic chemistry to describe the number of carbons attached to a specific atom, however, the atom will not always a carbon.
Example \
Please indicate the the number of 1o, 2o, 3o, and 4o carbons in the following molecule:
- The molecule has six Primary carbons .
- The molecule has one Secondary carbon .
- The molecule has one Tertiary carbon .
- The molecule has one Quaternary carbon
Hydrogen atoms are also classified in this manner. A hydrogen atom attached to a primary carbon atom is called a primary hydrogen ect.
Example \
Please indicate the the number of 1o, 2o, and 3o, hydrogens are in the following molecule:
- The molecule has fifteen Primary hydrogens.
- The molecule has two Secondary hydrogens.
- The molecule has one Tertiary hydrogen.
Read Also: Lewis Dot For Ccl4
Chemistry Is A Physical Science
Chemistry is typically considered a physical science, as defined by the Encyclopedia Britannica, because the study of chemistry does not involve living things. Most of the chemistry involved in research and development, such as making new products and materials for customers, falls within this purview.
But the distinction as a physical science becomes a bit blurry in the case of biochemistry, which explores the chemistry of living things, according to the Biochemical Society. The chemicals and chemical processes studied by biochemists are not technically considered “living,” but understanding them is important to understanding how life works.
Law Of Chemical Combination
7.1 Law of Conservation of Mass
In a chemical reaction the mass of reactants consumed and mass of the products formed is the same, that is mass is conserved. This is a direct consequence of the law of conservation of atoms. This law was given by Antoine Lavoisier in \.
7.2 Law of Constant / Definite Proportions
The law of definite proportions states that the mass proportions of the elements in a composite sample are always the same. It was given by a French chemist, Joseph Proust.
7.3 Law of Multiple Proportions
The law of multiple proportions states that when two elements are combined to form more than one compound, the weight of one element is proportional to the fixed weight of the other element as a whole number. This law was proposed by Dalton in \.
7.4 Law of Reciprocal Proportions
The law states that if two different elements are combined with the fixed mass of the third element, their combined mass ratio is the same, or a simple multiple of their combined mass. This Law was proposed by Richter in \ .
7.5 Gay Lussacs Law of Gaseous Volumes
The law developed by Gay Lussac in 1808 establishes that “the relationship between the volume of a gaseous reactant and a product can be represented by a simple whole number.”
7.6 Avogadro Law
In \ , Avogadro proposed that equal volumes of gases at the same temperature and pressure should contain an equal number of molecules.
You May Like: Unit 1 Test Geometry Basics Answers Key
Class 12 Chemistry Revision Notes For Chapter 3
Electrochemistry is a vital section of chemistry that determines the function of electrodes and reactors. Vedantus Electrochemistry notes class 12 tries to situate the ideas behind the chemical reactions.
An electrochemical cell is a tool that produces the difference between forms of the electrode through a chemical reaction. There are ideally two types of electron conductors that get separated by an ionic conductor. An electron conductor further links it, making it accessible.
Class 12 Chemistry Chapter 3 notes explain this function of electrons where two metallic electrodes are present. These metallic electrodes are immersed in an electrolytic solution for power generation. By thorough reading of chapter 3 Chemistry class 12 notes, students will know that the ionic conductor is a vital part of cells.
Study without Internet
What Is Inorganic Chemistry
The word organic refers to the compounds which contain the carbon atoms in it. So the branch of chemistry that deals with the study of compounds, which does not consist of carbon-hydrogen atoms in it, is called Inorganic Chemistry. In simple words, it is opposite to that of Organic Chemistry. The substances which do not have carbon-hydrogen bonding are the metals, salts, chemical substances, etc.
On this planet, there are known to exist about 100,000 number of Inorganic compounds. Inorganic chemistry studies the behaviour of these compounds along with their properties, their physical and chemical characteristics too. The elements of the periodic table except for carbon and hydrogen, come in the lists of Inorganic compounds.
Many of the elements are technologically important: titanium, iron, nickel and copper, for example, are used structurally and electrically. Second, the transition metals form several useful alloys, with each other and with other metallic elements.
Recommended Reading: How To Find Ksp From Solubility
What Does Ch Stand For Organic Chemistry
We compiled queries of the CH abbreviation in Organic Chemistry in search engines. The most frequently asked CH acronym questions for Organic Chemistry were selected and included on the site.
We thought you asked a similar CH question to the search engine to find the meaning of the CH full form in Organic Chemistry, and we are sure that the following Organic Chemistry CH query list will catch your attention.
Applications Of Inorganic Chemistry
Inorganic chemistry finds its high number of applications in various fields such as Biology, chemical, engineering, etc
- It is applied in the field of medicine and also in healthcare facilities.
- The most common application is the use of common salt or the compound Sodium hydroxide in our daily lives.
- Baking soda is used in the preparation of cakes and other foodstuffs.
- Many inorganic compounds are utilized in ceramic industries.
- In the electrical field, it is applied to the electric circuits as silicon in the computers, etc.
Recommended Reading: Algebra 1 Eoc Test Answers
Examples Of Chemistry In A Sentence
chemistrychemistrychemistrychemistry clevelandchemistry Washington Postchemistry Detroit Free Presschemistry CNNchemistrySan Diego Union-Tribunechemistry Chronchemistry BostonGlobe.comchemistryUSA TODAY
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘chemistry.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
What Element Is Ch On The Periodic Table

4.8/5
| Atomic number |
|---|
Also know, what is CH on the periodic table?
The Internet is a great place to find periodic tables that contain additional information. One item on most periodic tables is the atomic mass of each element. Appendix: Periodic Table of the Elements.
| Name | |
|---|---|
| Footnotes | g, r |
Furthermore, is a chemical element with symbol? In chemistry, a symbol is an abbreviation for a chemical element. Symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised.
Also know, what does be stand for in the periodic table?
Periodic Table with Element Names and Electronegativity
| Element Name |
|---|
What is the name of 119 Element?
eka-francium
Read Also: Half Life Chemistry Formulas