Next Up: Molecular Orbital Theory
What will help us answer these questions, as well as many others going forward is a concept called molecular orbital theory.
In the following posts in this series, were going to dig more deeply into how p orbitals overlap to form molecular orbitals, and well examine the energy levels of these orbitals. Well also see how this influences the reactivity of molecules and allows us to make predictions about their chemical behaviour.
As well see, molecular orbital theory provides us with a very powerful set of concepts that will help us understand chemical reactivity at a much deeper level.
Thanks to Tom Struble for all his help with this post.
Determine A Target Chemical Moiety On Your Prosthetic Molecule
Conjugated proteins are proteins linked with chemical groups on other molecules, called prosthetic molecules. Prosthetic molecules can be carbohydrates, lipids or even ions.
You need to determine which chemical moiety on your prosthetic molecule you will conjugate to the protein of interest using the bifunctional reagent.
The best moieties on your prosthetic molecule to bind to are amines, carboxyl groups, or sulfhydryls as we stated previously.
If you can install an azide, tetrazine, or alkyne on your prosthetic molecule, these groups can make your protein conjugation chemistry really easy.
If none of these groups are available on your prosthetic molecule, consider converting a hydroxyl to an aldehyde to make it more reactive.
Typically nanoparticles that are commercially purchased can include functionalized polymer surfaces that you can conjugate to proteins. We discuss protein and antibody bioconjugation to gold in our article.
Azide-alkyne click chemistry is a common method utilized for protein conjugation chemistry. Its considered bioorthogonal because azides and alkynes are uncommon in proteins. Image from Royal Society of Chemistry.
Learn more about orthogonal bioconjugation techniques in our related article.
Bifunctional Reagents For Protein Conjugation Chemistry
A bifunctional reagent features reactive or some very active end groups or molecules that make bioconjugation possible. These bifunctional reagents can actively bind to compatible functional groups through their active end groups.
Research has shown four main functional groups make effective target moieties on proteins and prosthetic molecules. These functional groups include
- Primary amines: A primary amine has an amino functional group . We can have the aliphatic amine and the Aromatic amine . Aromatic amines are more reactive and easier to conjugate to proteins than aliphatic amines.
- Carbonyls: Identified by the carbonyl functional group .
- Thiols : This is a functional group that has sulfur bonded to a hydrogen atom. They are usually denoted as R-SH.
- Carboxyls: Denoted RCOOH.
A closer look at all four bifunctional groups shows that they are also a part of amino acids, so they are easy to find on proteins.
Some common homo and hetero bifunctional reagents for protein conjugation. Image from SpringerLink.
Read Also: Are Michael Jackson’s Children Biologically His
Advantages Of Maleimide Protein Conjugation
- Thiols are only present in proteins on Cysteines
- For many proteins, one or two disulfide bonds exist, which mean that you can reduce them and conjugate in a site-selective manner using thiol-maleimide chemistry
- Cysteines can be readily introduced into proteins without affecting function, using site directed mutagenesis
Here are some strategies for using thiol-maleimide protein conjugation chemistry to create cleavable and permanent antibody drug conjugates. Image from RSC.
Molecular Orbitals Of 13 Dienes
A molecular orbital model for 1,3-butadiene is shown below. Note that the lobes of the four p-orbital components in each pi-orbital are colored differently and carry a plus or minus sign. This distinction refers to different phases, defined by the mathematical wave equations for such orbitals. Regions in which adjacent orbital lobes undergo a phase change are called nodes. Orbital electron density is zero in such regions. Thus a single p-orbital has a node at the nucleus, and all the pi-orbitals shown here have a nodal plane that is defined by the atoms of the diene. This is the only nodal surface in the lowest energy pi-orbital, 1. Higher energy pi-orbitals have an increasing number of nodes.
- This page has no tags.
Read Also: What Is Figure Ground Perception Psychology
Protein Conjugation Protocol Example
There are several protein conjugation kits that are available. Heres an example from Vector labs. However, you can also utilize the chemical reactions above to create protein conjugates.
The steps below show a typical example of conjugating to lysines on a protein, based on information from GBiosciences.
Conjugated System Facts For Kids
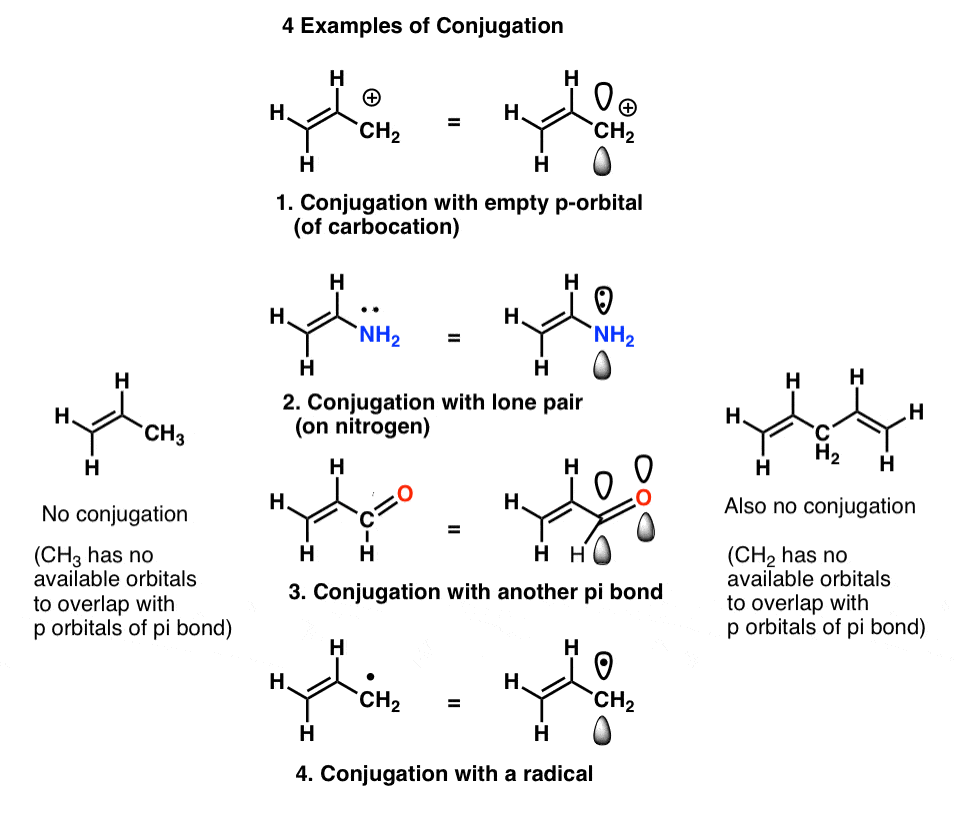
In chemistry, a conjugated system is a system of connected p-orbitals with delocalized electrons. Conjugated systems are created by several multiple bonds, each separated by single bonds. In general, conjugated systems may lower the overall energy of the molecule and increase how steady it is. They can contain lone pairs, radicals or carbenium ions. The compound may be cyclic, acyclic, linear or mixed. In most cases, the atoms in a molecule are held together by single bonds . Molecules which have a conjugated system have unique properties different from normal compounds created by the sharing of the delocalized electrons among many atoms.
Conjugation is the overlap of one p-orbital with another across a sigma bond that is in between. .
A conjugated system has a region of overlapping p-orbitals, bridging the single bonds that are between. They allow a delocalization of pi electrons across all the adjacent aligned p-orbitals. The pi electrons do not belong to a single bond or atom, but rather to a group of atoms.
The largest conjugated systems are in graphite, conductive polymers, and carbon nanotubes.
Also Check: Exponential Growth And Decay Worksheet Answer Key
Maleimide Protein Conjugation Reaction Chemistry
You can react cysteines in proteins with maleimide groups as long as your buffer is in a pH between 6.0 and 7.0. A common approach is to reduce disulfide bonds in proteins first with DTT or TCEP and then to react with a maleimide-containing linker.
At a pH between 6.0 to 7.0, the maleimide group can react with sulfhydryl groups resulting in the production of a stable thioether linkage and the reaction is irreversible. At pH above 8.0 , say 8.5, the reaction seems to favor primary amine and the rate of hydrolysis of the maleimide group to maleimide acid will increase.
You must eliminate compounds that contain thiol groups, like dithiothreitol and -mercaptoethanol , from the reaction because they tend to compete with thiol groups on proteins. For instance, if you utilized dithiothreitol to reduce disulfides in a protein and open up the sulfhydryl groups, you would need to thoroughly remove the DTT using a desalting column before you start the maleimide reaction. Instead of DTT, consider using the disulfide-reducing agent TCEP which lacks thiols.
You can quench free maleimide left over after reacting your protein by adding free thiols. You can also check your conjugation reactions using our HPLC Step By Step guide.
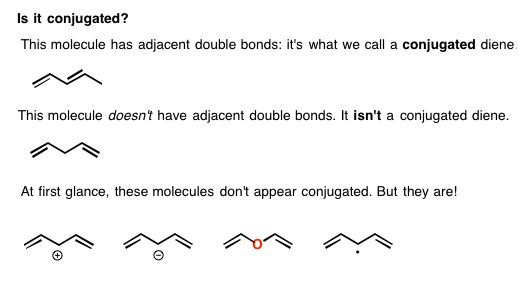
The Distinction Between Conjugation And Resonance
You might well ask: this just sounds like resonance. Whats the difference?
Lets take a second to distinguish conjugation and resonance.
- Conjugation is what we call it when 3 or more p orbitals join together into a larger pi system.
- These conjugated pi systems contain electrons, which we often call pi electrons to distinguish them from the electrons that comprise single bonds in the molecule.
- The different arrangements of electrons within that pi system are called resonance forms.
A rough analogy could go like this:
- Think of p orbitals as being a bit like rooms for electrons
- Joining several rooms together into a larger building is conjugation
- The different allowable arrangements of people within that building are resonance forms.
The key requirement for conjugation is orbital overlap, which well expand on in a bit.
For now, lets review some consequences of conjugation.
Don’t Miss: My Hrw Answers Algebra 1
Polymersmall Molecule Drug Conjugates
Fig. 6. Polymer conjugate architectures. Chain-end conjugation by chemoselective initiator/chain transfer agent or by postmodification of polymer chain-end . Conjugation via reactive functional groups along the polymer side chain.
Fig. 7. Reactive side chains of polymers by controlled radical polymerization for drug conjugation: N-hydroxysuccinimide ester, p-nitrophenyl ester, pentafluorophenyl ester, epoxide, oxazolone, azide, alkyne, trimethylsilyl propargyl, pyridyl disulfide, acetals which are easily converted to reactive aldehydes.
Fig. 8. Structure of polymer conjugates of tamoxifen,107 camptothecin,108 and doxorubicin.109
Geetha Ramachandran, Soumya Swaminathan, in, 2014
Polymerdrug Conjugates For Cancer Treatment
The basic carrier design and construction of the drug carrier represents a pivotal task in polymerdrug conjugation in terms of solubility and steric accessibility, as well as reactivity, of the drug-anchoring sites . The basic carrier design consists of a linear, biofissionable polymer structure comprising subunits equipped with hydrosolubilizing moiety and other subunits providing functional groups suitable for drug binding. Since the solubility of the conjugate is a determining factor in polymerdrug conjugation, as a general trend, the designed carrier contains more than 50% of the hydrosolubilizing moieties. In simple terms, the carrier polymer acts as a vehicle transporting the bioactive agent. This system, that is, conjugate, is administered intravenously, circulates in different parts of the body and delivers the active agent in the target cells . Water-soluble polyamides based on poly and PAA structures, as well as ester-amine have been extensively studied and their conjugates tested for different types of cancers for both in vitro and in vivo. Bioactive agents, such as platinum containing agents, ferrocene complexes, and methotrexate have been used.
F.M. Veronese, G. Pasut, in, 2007
PEG is often the polymer of choice for protein conjugation, being monofunctional, non-toxic, non-immunogenic, non-antigenic and highly soluble in water.
Ameeta Parekh, in, 2012
Don’t Miss: Geometry Basics Segment Addition Postulate Worksheet Answers
Amplified Fluorescent Conjugated Polymers As Sensors
Conjugated polymers designed and used for explosive detection rely on fluorescence signals and events to indicate presence of an analyte of interest . Fluorescence spectroscopy is inherently a very sensitive technique, and very small changes in fluorescence intensity can be reliably detected. To induce fluorescence, the material is typically irradiated with a frequency of ultraviolet or visible light that it can absorb. This promotes an electron from the valence band into the conduction band, forming a bound electronhole pair known as an exciton. Some of these excitons relax to the ground state in a radiative fashion by emitting light as fluorescence, whereas the rest of them release their energy non-radiatively as heat in the form of molecular motion. In between these two events, absorption and emission, is a very short but important time during which the molecular wire nature of conjugated polymers plays a critical role.
Figure 2. Contrasting monomeric fluorescent receptors and those wired in series.
To summarize, absorption of a photon by a conjugated polymer creates an exciton that can then sample many potential binding sites within its lifetime. Emission from the excited state is observed only if there is no bound analyte encountered by the migrating exciton. If the analyte is encountered there is quenching of the emission, and it is this amplified dimunition in emission intensity that serves as an indication that the analyte is present.
Find A Bifunctional Reagent That Can React With Both Chemical Moieties
Bifunctional reagents are important in the protein conjugation process as weve discussed previously.
The bifunctional reagent that you use to conjugate your protein to the prosthetic molecule must include chemical groups that can react with your protein and chemical groups that can react with your prosthetic molecule .
If the two groups from Step 1 and Step 2 above are different, you need to use a heterobifunctional reagent. Western blotting can be utilized to prove that your conjugation reactions yielded higher molecular weight results.
Bifunctional reagents are also really useful for attaching proteins and other molecules to surfaces.
Using heterobifunctional reagents for protein conjugation chemistry
To use a heterobifunctional reagent, first react it with the protein. This reduces steric hindrance compared to the opposite case where you first react with the prosthetic group because your linker bifunctional reagent will be able to reach deeper parts of your protein without the prosthetic group interacting with the protein.
After reacting with the protein, you can react to the other functional group on the bifunctional reagent to react with your prosthetic molecule.
Note that two steps are involved for heterobifunctional reagents in order to avoid polymerization or side linkage of functional groups.
Using homobifunctional reagents for protein conjugation
Site-specific protein conjugation
Bioorthogonal reactions for protein conjugation
Read Also: What Is The Molecular Geometry Of Ccl4
Bridgehead Amides Are Not Conjugated And Are Much More Easily Broken Than Ordinary Amides
Bridgehead amides give an illustration of what happens to amides when overlap is impossible.
Just as we saw in bridgehead alkenes, in bridgehead amides, orbital overlap between the nitrogen lone pair and carbonyl carbon is impossible due to twisting. The result is that the C-N bond does NOT have partial double bond character and it is much easier to break than a normal amide.
The bridgehead amide below is quinuclidone, a twisted amide that eluded synthesis for decades. It was only in 2006 that it was finally made through a clever route by the lab of Brian Stoltz at Caltech.
The X-ray crystal structure bears witness to the lack of conjugation in this amide. The C-N bond length is 1.52 Å and the C=O bond length is 1.19 Å, which is typical of a bond length in an aldehyde or ketone . We would therefore expect that it is quite a bit more unstable towards nucleophilic attack than a normal amide, which was borne out in the Stoltz labs study.
Porphyrins And Similar Compounds
Porphyrins have conjugated molecular ring systems that appear in many enzymes of biological systems. As a ligand, porphyrin forms numerous complexes with metallic ions like iron in hemoglobin that colors blood red. Hemoglobin transports oxygen to the cells of our bodies. Porphyrinmetal complexes often have strong colors. A similar molecular structural ring unit called chlorin is similarly complexed with magnesium instead of iron when forming part of the most common forms of chlorophyll molecules, giving them a green color. Another similar macrocycle unit is corrin, which complexes with cobalt when forming part of cobalamin molecules, constituting Vitamin B12, which is intensely red. The corrin unit has six conjugated double bonds but is not conjugated all the way around its macrocycle ring.
Also Check: What Does The Denominator Tell You
What Is Conjugated System Of Pi Bond
A conjugated system is a system of connected p-orbitals with delocalized electrons in compounds with alternating single and multiple bonds, which in general may lower the overall energy of the molecule and increase stability. They allow a delocalization of pi electrons across all the adjacent aligned p-orbitals.
What Is The Concept Of Conjugate In Acid
A conjugate means a mate. If we translate this meaning to acid-base chemistry, then we can say that every acid is tied to its mate called conjugate base, and together, they are called a conjugate acid-base pair. Similarly, every base is tied to its mate called conjugate acid, and together, they are called a conjugate acid-base pair. Therefore, every Brønsted-Lowry acid has its conjugate base. And every Brønsted-Lowry base has its conjugate acid. Lets further explain this using the following model:
From the model, you can see that a conjugate acid is related to its conjugate base by the loss of a hydrogen ion, while the conjugate base is related to its conjugate acid by the gain of a hydrogen ion. That is a conjugate acid and its conjugate base are related by the loss and gain of a hydrogen ion. For instance, when a conjugate acid donates a hydrogen ion, it turns into its conjugate base. And when a conjugate base accepts a hydrogen ion, it turns into its conjugate acid.
Chemists sometimes call a hydrogen ion a proton. This is because a hydrogen atom, which has only one electron and one proton, becomes a positive hydrogen ion only when it loses its single electron. And when it does, it becomes a hydrogen ion with only one proton. For this reason, a hydrogen ion is the same as a proton.
Now lets chemically illustrate conjugate acid-base pairs using the following example:
You May Like: What Is The Molecular Geometry Of Ccl4
Conjugated Vs Nonconjugated Dienes
Conjugated dienes are two double bonds separated by a single bond
Nonconjugated Dienes are two double bonds are separated by more than one single bond.
When using electrostatic potential maps, it is observed that the pi electron density overlap is closer together and delocalized in conjugated dienes, while in non conjugated dienes the pi electron density is located differently across the molecule. Since having more electron density delocalized makes the molecule more stable conjugated dienes are more stable than non conjugated
For example in 1,3-butadiene the carbons with the single bond are sp2 hybridized unlike in nonconjugateddienes where the carbons with single bonds are sp3 hybridized. This difference in hybridization shows that the conjugated dienes have more ‘s’ character and draw in more of the pi electrons, thus making the single bond stronger and shorter than an ordinary alkane C-C bond .
Consequences Of Conjugation : Bond Lengths
As I just said, were more used to conjugation in the context of resonance, a concept weve covered before
For instance, with the acetate ion and the allyl cation , we saw that theres two different ways of arranging the pi electrons, which we call resonance forms.
The important point to note is that the pi-electrons in these are not constantly switching back-and-forth between atoms rather, the true structure of the molecule is a hybrid of these resonance forms.
One important consequence of resonance is bond lengths that are intermediate between two forms.
For example, the C-O bond length in the acetate ion is between what wed expect for a C-O pi bond and a C-O single bond .
Recommended Reading: How To Find Ksp Chemistry
Effect On Chemical Properties
Hyperconjugation affects several properties.
- 3C+> 2CH+> CH2+> CH3+
- The three CH bonds of the methyl group attached to the carbocation can undergo the stabilization interaction but only one of them can be aligned perfectly with the empty p-orbital, depending on the conformation of the carboncarbon bond. Donation from the two misaligned CH bonds is weaker. The more adjacent methyl groups there are, the larger hyperconjugation stabilization is because of the increased number of adjacent CH bonds.