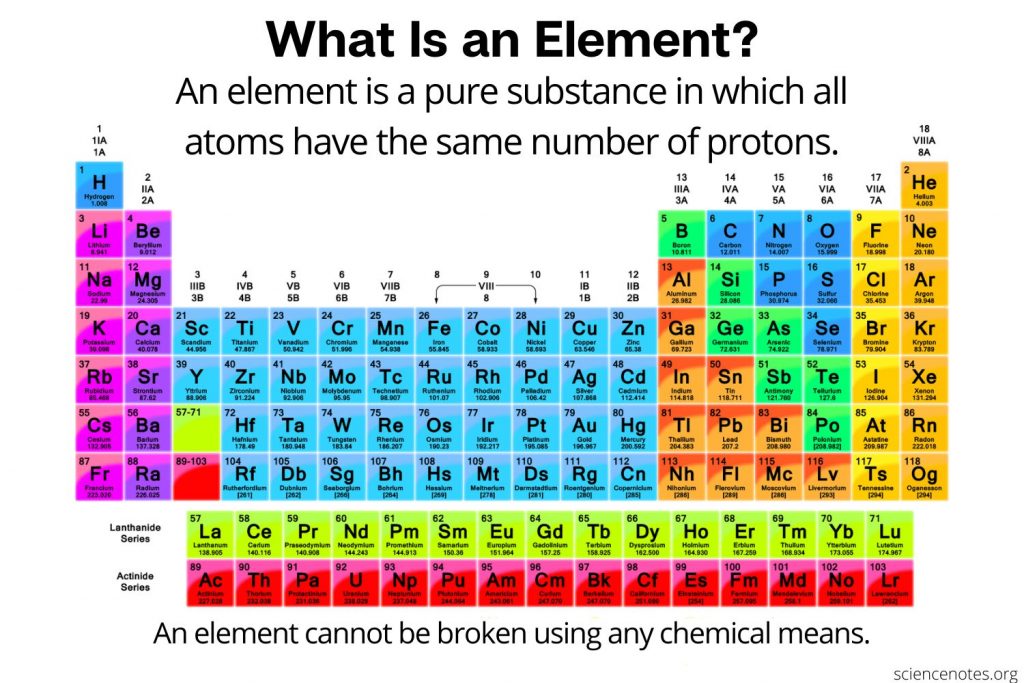
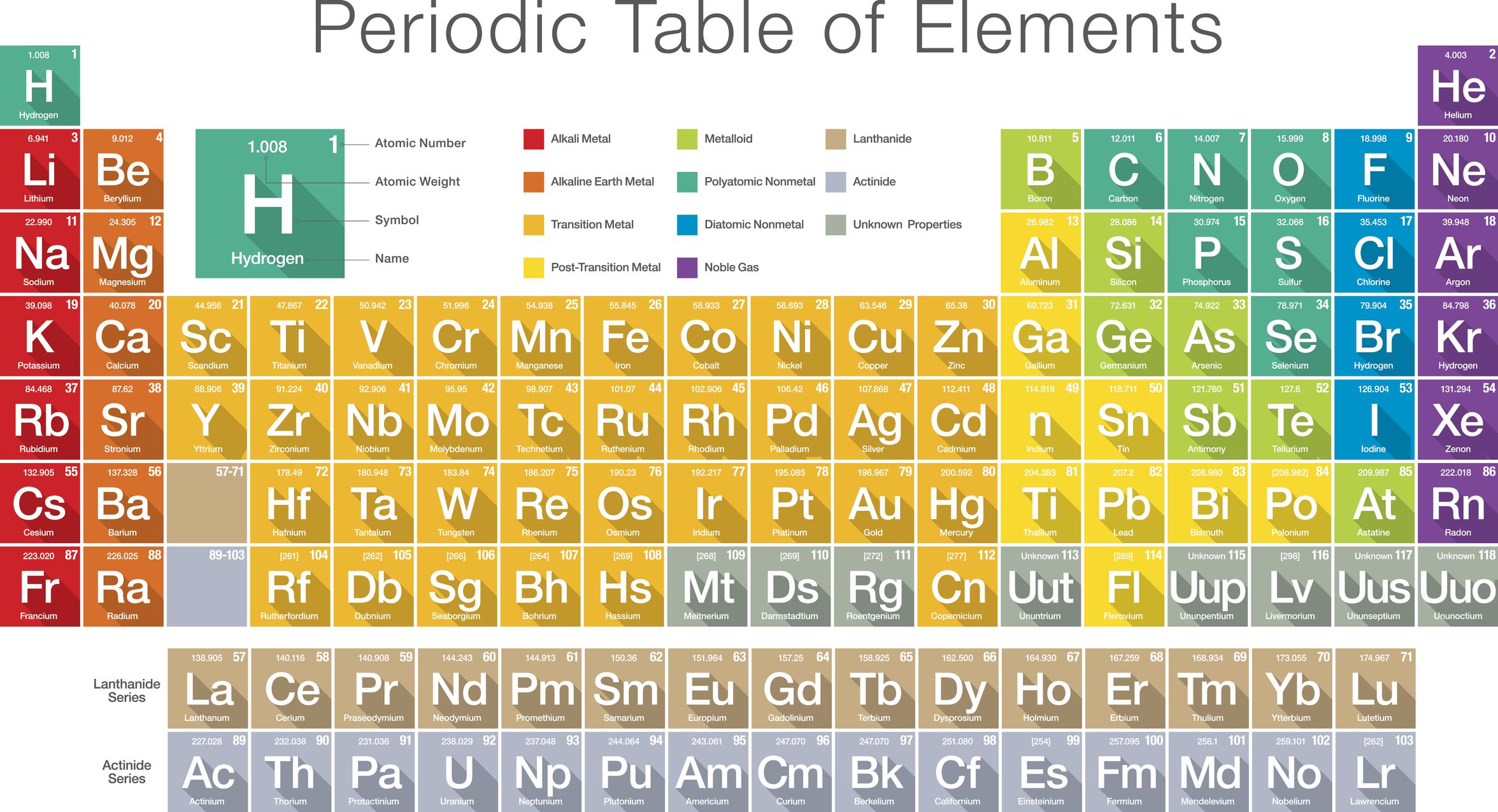
How Is The Periodic Table Arranged
The periodic table is arranged by atomic weight and valence electrons. These variables allowed Mendeleev to place each element in a certain row and column . The table comprises seven rows and 18 columns. Each element in the same row has the same number of atomic orbitals as the others in that row or period. That means all of the elements in the third period sodium, magnesium, aluminum, silicon, phosphorus, sulfur, chlorine and argon have three atomic orbitals where their electrons reside. Meanwhile, the column or group signifies the number of electrons in the atom’s outermost shell these are called the valence electrons, and they are the electrons that can chemically bond with valence electrons of other elements. The valence electrons can be either shared with another element, a type of covalent bonding, or exchanged in a type of ionic bonding, according to Lumen Learning .
For example, all of the elements in the second column have two valence electrons in the third column, they have three valence electrons. There are some exceptions to this rule in the transition elements, which fill the shorter columns at the center of the periodic table. These transition elements
Representing The Direction Of The Chemical Reaction
The reactants and the products can be separated by one of the following four symbols.
- In order to describe a net forward reaction, the symbol is used.
- In order to describe a state of chemical equilibrium, the symbol is used.
- To denote stoichiometric relationships, the = symbol is used.
- In order to describe a reaction that occurs in both forward and backward directions, the symbol is used.
Multiple entities on either side of the reaction symbols described above are separated from each other with the help of the + symbol in a chemical equation. It can be noted that the symbol, when used in a chemical equation, is often read as gives rise to or yields.
How Is The Periodic Table Used Today
By knowing that certain elements that are lumped together on the table have certain characteristics and behaviors, scientists can figure out which ones would be best for certain industries and processes. For instance, engineers use different combinations of elements in Groups III and V of the table to create new semiconductor alloys, such as gallium nitride and Indium nitride , according to the National Institute of Standards and Technology .
In general, chemists and other scientists can use the table to predict how certain elements will react with one another. The alkali metals, for instance, are in the first column or group of the table and tend to have one valence electron and so carry a charge of +1. This charge means they “react vigorously with water, and combine readily with nonmetals,” chemist Anne Marie Helmenstine wrote on ThoughtCo. Magnesium, which is in the same group on the table as calcium, is becoming useful as part of alloys for bone implants, NIST said. Since these alloys are biodegradable, they serve as a scaffolding and then disappear after natural bone grows on the structures.
Additional reporting by Traci Pedersen, Live Science contributor
Recommended Reading: College Algebra Practice Problems And Solutions
What Is A Chemical Example
Chemical substances are often called pure to set them apart from mixtures. A common example of a chemical substance is pure water it has the same properties and the same ratio of hydrogen to oxygen whether it is isolated from a river or made in a laboratory.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Origin Of The Concept
Walther Kossel proposed a theory in which a total transfer of electrons occurred.
Since ancient times it has been suspected that the constituent elements of matter are grouped together according to certain forms of affinity. But it would be in 1704 when Isaac Newton would postulate his theory of chemical bonding , based on the atomic theories already formulated, and noting that a force held the atoms together in the observed chemical reactions.
In 1819, thanks to the invention of the voltaic pile, Jöns Jakob Berzelius introduced the notions of electropositivity and electronegativity to the theory of atomic combination, and later, in the 20th century , Gilbert Lewis introduced the concept of the bond formed by pair of electrons , giving rise to the possibility of single, double and triple bonds, since atoms could share between one and six electrons. Thus the covalent bonds arose.
In 1927 a theory similar to that of Lewis also emerged , but in which a total transfer of electrons occurred, and it was proposed by Walther Kossel. This would give rise to ionic bonds.
The above content published at Collaborative Research Group is for informational and educational purposes only and has been developed by referring reliable sources and recommendations from technology experts. We do not have any contact with official entities nor do we intend to replace the information that they emit.
Read Also: Ratios And Proportions Algebra 1
Who Created The Periodic Table
Dmitri Mendeleev, a Russian chemist and inventor, is considered the “father” of the periodic table, according to the Royal Society of Chemistry . In the 1860s, Mendeleev was a popular lecturer at a university in St. Petersburg, Russia. At the time, no modern organic chemistry textbooks in the Russian language existed, so Mendeleev decided to write one. As he was working on that book, titled “Principles of Chemistry” , he simultaneously tackled the problem of the disordered elements, according to Khan Academy .
Putting the elements in any kind of order would prove quite difficult. At the time, there were 63 known chemical elements, each with an atomic weight calculated using Avogadro’s hypothesis, which states that equal volumes of gases, when kept at the same temperature and pressure, hold the same number of molecules.
Just two strategies existed at the time to categorize these elements: separating them into metals and nonmetals or grouping them by an element’s number of valence electrons . The first section of Mendeleev’s book dealt with just eight of the known elements carbon, hydrogen, oxygen, nitrogen, chlorine, fluorine, bromine and iodine and those two strategies worked for those particular elements, according to Michael D. Gordin in his book “A Well-Ordered Thing: Dmitrii Mendeleev and the Shadow of the Periodic Table” . But they weren’t enough to usefully sort the 55 additional chemical elements known at the time.
Key Takeaways: Chemical Element Definition
- A chemical element is a substance that cannot be further broken down by any chemical reaction.
- Each element has a unique number of protons in its atom. For example, a hydrogen atom has 1 proton, while a carbon atom has 6 protons.
- Varying the number of electrons in an atom of an element produces ions. Changing the number of neutrons produces isotopes.
- There are 118 known elements.
You May Like: How Many Biological Children Does Mia Farrow Have
How Elements Came To Be Defined Correctly
In 1913, chemistry and physics were topsy-turvy. Some big hitters – including Dmitri Mendeleev – were talking seriously about elements lighter than hydrogen and elements between hydrogen and helium. Visualizing the atom was a free-for-all, and Mendeleev’s justification for a periodic table based on the elements’ atomic weights was falling apart at the seams.
This is the story of how Henry Moseley brought light to the darkness.
Periods Groups And The Periodic Table
The periodic table is a commonly used method of organizing the elements that provides useful information about the elements and their behavior. In Fig. 2.12, elements in blue are metals and elements in yellow are nonmetals. In Figure 2.13, the entry for hydrogen highlights the placement of the atomic number, element symbol, element name, and atomic weight.
The periodic table has three prominent features. First, the periodic table is arranged in horizontal rows, which are called periods. There are seven periods. In Period 1 there are two elements, hydrogen and helium . The second and third periods both contain eight elements, the fourth and fifth periods contain 18 elements, and the sixth and seventh periods contain 32 elements.
Second, all of the elements are listed sequentially according to their atomic numbers. The atomic number corresponds to the number of protons and is found above the elements symbol. For example, in Figure 2.13, the atomic number of hydrogen is 1, found over the H.
Don’t Miss: What Ph Stands For In Chemistry
How Many Elements Are There
There are currently 118 elements known to humankind. 94 elements are naturally occurring, and 24 elements have been artificially produced, or synthetically produced, via nuclear reactions. These 24 elements are sometimes called man-made elements. Elements can be solids, liquids or gases at room temperature, 20 degrees Celsius.
The first element on the periodic table is hydrogen. The last element, the one most recently synthesized in 2002, is element 118, oganesson named after the Russian-Armenian physicist Yuri Oganesson.
Out of the 94 naturally occurring elements, 6 of them occur only in extremely small trace quantities for example francium or neptunium. Those six are all radioactive and only exist as decay products of other more abundant radioactive elements.
Compounds Containing A Metal Ion With A Variable Charge
Most of the transition metals can form two or more cations with different charges. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+ , and the two corresponding compound formulas are FeCl2 and FeCl3. The simplest name, iron chloride, will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron chloride and iron chloride, respectively. Other examples are provided in Table 4.
| Table 4. Names of Some Transition Metal Ionic Compounds | |
|---|---|
| Transition Metal Ionic Compound | |
| SnF4 | tin flouride |
Read Also: Do You Need Physics For Med School
Double And Triple Bonds
Double bonds are graphically represented by two lines.
Covalent bonds can be single, double or triple, depending on the number of electronic pairs shared between the atoms that compose them . The more electrons that are shared, the stronger the bond will be and the more energy it will cost to break it.
Single bonds are graphed with a single AA line, double bonds with two A = A, and triple bonds with three A?A.
Element Names Symbols And Atomic Numbers
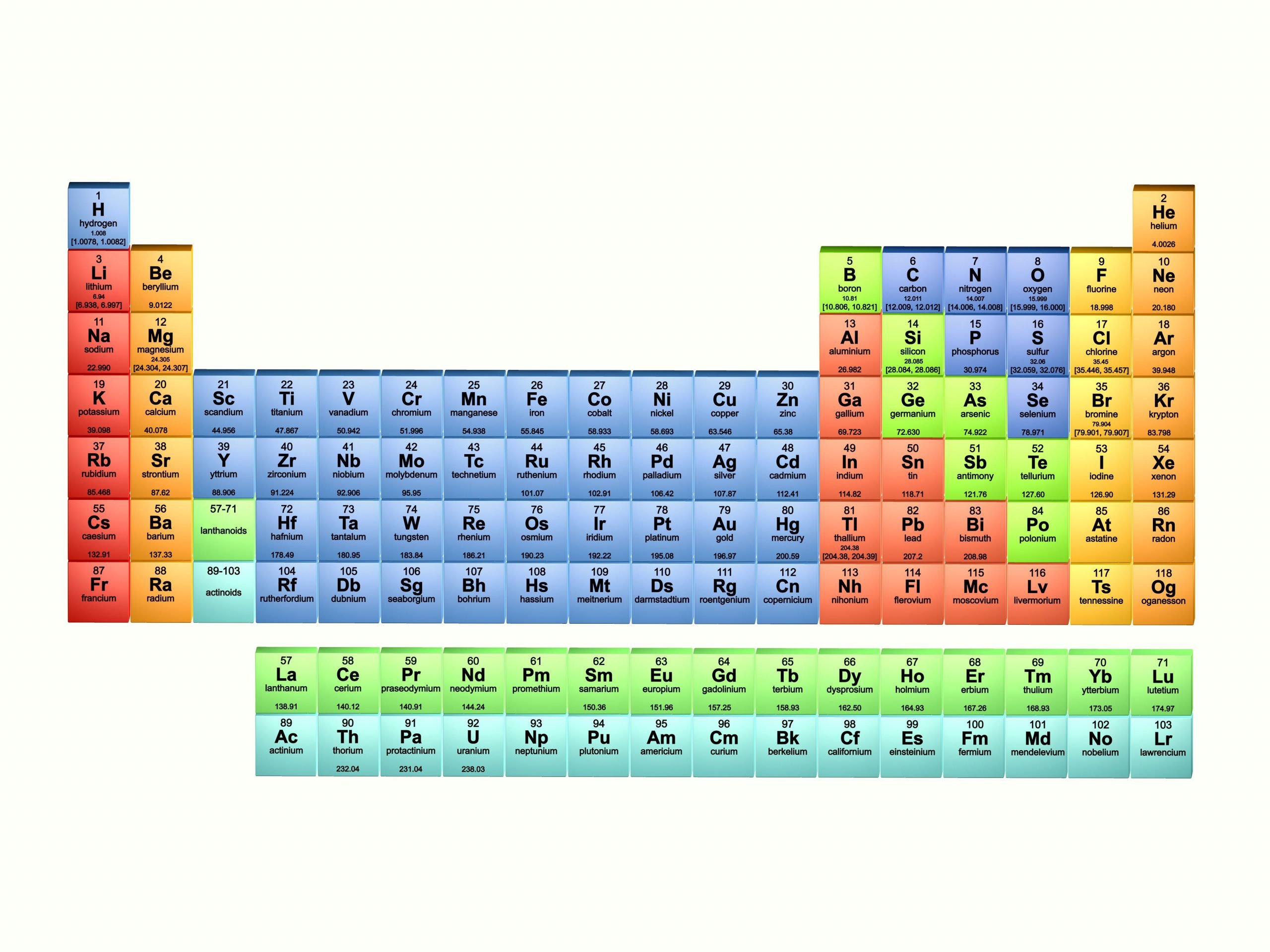
There are three ways to refer to individual elements. Each element has a name, an element symbol, and an atomic number. The International Union of Pure and Applied Chemistry approves standard names and symbols, but within an individual country, other element names might be used.
Some element names are historical, but most were named by the person or group who discovered them. Element names usually reference a person , place , or mineral. Many element names end with the -ium suffix, but halogen names have the -ine ending and noble gases have the -on ending. An element name refers to a single atom or ion of that element, its isotopes, or to a molecule consisting only of that element. For example, oxygen may refer to a single oxygen atom, oxygen gas , or the isotope oxygen-18.
Each element also has a unique one- or two- letter symbol. Examples of symbols include H for hydrogen, Ca for calcium, and Og for oganesson.
The periodic table lists the elements in order of increasing atomic number. The atomic number is the number of protons in any atom of that element. Examples of atomic numbers include 1 for hydrogen, 2 for helium, and 6 for carbon.
Read Also: Three Dimensional Geometry Class 12 Important Questions
Examples Of Chemical Properties
Examples of chemical properties of a substance can include:
- Toxicity
- Chemical stability under specific conditions
- Acidity or basicity
- Radioactivity
Remember, a chemical change must occur for a chemical property to be observed and measured. For example, iron oxidizes and becomes rust. Rusting is not a property that can be described based on analysis of the pure element.
Characteristics Of A Chemical Bond
Polarity
If two atoms of different elements are linked, a polar molecule could form.
Polarity is a characteristic of molecules formed by covalent bonds, and it depends directly on the nature of the bonded atoms .
If two atoms of the same element are bonded , there will be no difference between the electronegativity of each. In this way, the pair of electrons they share will be attracted to each of these atoms with the same force, so the distribution of electrical charges will be uniform in the molecule they form.
The same happens when atoms of different elements are bonded , but whose electronegativity difference is very small . Molecules formed in this way are called nonpolar or apolar and this type of bonding nonpolar covalent bond.
On the other hand, if two atoms of different elements are bonded , and the electronegativity difference between them is greater than 0.4 , then the most electronegative atom attracts towards itself with greater force the electrons of the bond, generating a non-uniform charge distribution in the structure. of the molecule they form. The molecules formed by this process are called polar and this type of bond is polar covalent bond.
Electrovalence
Electrovalence occurs when an atom gains or loses electrons.
Ionic bonds are characterized by the fact that their atoms have a large electronegativity difference , to the extent that one loses and the other receives electrons when joining.
Read Also: How To Do Well In Math Class
Uses Of Chemical Properties
Chemical properties are of great interest to materials science. These characteristics help scientists classify samples, identify unknown materials, and purify substances. Knowing the properties helps chemists make predictions about the type of reactions to expect. Because chemical properties are not readily apparent, they are included in labels for chemical containers. Hazard labels based on chemical properties should be affixed to containers, while full documentation should be maintained for easy reference.
Chemical Energy In Everyday Life
- We know that plants need solar energy to produce sugar from carbon dioxide to water. Sugar, water and carbon dioxide stay together by chemical bonds that hold the chemicals together.
- For instance, all sugars consist of oxygen, carbon and hydrogen atoms held together by chemical bonds. These atoms do not connect together automatically rather some energy is required to make them stay together.
- Plants use solar energy to put the hydrogen, carbon and oxygen atoms as a whole in the form of sugar. This is a suitable example of energy transformation where energy is transformed from one form to another. Here, solar energy is transformed into chemical energy and prevents it from falling apart.
Also Check: What Is An Intervening Obstacle In Human Geography
What Are The Main Elements
The Five Base Elements are Fire, Earth, Metal, Water, and Wood. In a state of continuous interaction and flux with each other these elements are known as different types of energy. Not only does The Five Elements mean Fire, Earth, Water, Metal, and Wood. We also apply to motion, transition, and growth.
What Are The 2 Main Types Of Elements
This is also possible to classify the elements into two major classes, the metals and non-metals. Metals usually have a translucent shiny sheen which is malleable, bendable and conducts electricity. Typically, nonmetals do not exhibit these properties.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Also Check: Geometry 2nd Grade Common Core Worksheets
Compounds Composed Of Two Elements
When two nonmetallic elements form a molecular compound, several combination ratios are often possible. For example, carbon and oxygen can form the compounds CO and CO2. Since these are different substances with different properties, they cannot both have the same name . To deal with this situation, we use a naming method that is somewhat similar to that used for ionic compounds, but with added prefixes to specify the numbers of atoms of each element. The name of the more metallic element is first, followed by the name of the more nonmetallic element with its ending changed to the suffix ide. The numbers of atoms of each element are designated by the Greek prefixes shown in above in Table 5.
When only one atom of the first element is present, the prefix mono is usually deleted from that part. Thus, CO is named carbon monoxide, and CO2 is called carbon dioxide. When two vowels are adjacent, the a in the Greek prefix is usually dropped. Some other examples are shown in Table 6.
| Table 6. Names of Some Molecular Compounds Composed of Two Elements |
|---|
| Compound |
Discovery And Recognition Of Various Elements
Ten materials familiar to various prehistoric cultures are now known to be chemical elements: Carbon, copper, gold, iron, lead, mercury, silver, sulfur, tin, and zinc. Three additional materials now accepted as elements, arsenic, antimony, and bismuth, were recognized as distinct substances prior to 1500 AD. Phosphorus, cobalt, and platinum were isolated before 1750.
Most of the remaining naturally occurring chemical elements were identified and characterized by 1900, including:
- The more common radioactive elements, including uranium, thorium, radium, and radon
Elements isolated or produced since 1900 include:
- The three remaining undiscovered regularly occurring stable natural elements: hafnium, lutetium, and rhenium
- Plutonium, which was first produced synthetically in 1940 by Glenn T. Seaborg, but is now also known from a few long-persisting natural occurrences
- The three incidentally occurring natural elements , which were all first produced synthetically but later discovered in trace amounts in certain geological samples
- Four scarce decay products of uranium or thorium , and
- Various synthetic transuranic elements, beginning with americium and curium
Don’t Miss: Chapter 12 Test Form 2b Geometry Answers