Molecular Chaperones Help Guide The Folding Of Many Proteins
The folding of many proteins is made more efficient by a special class of proteins called molecular chaperones. The latter proteins are useful for cells because there are a variety of different paths that can be taken to convert the molten globule form of a to the protein’s final compact . For many proteins, some of the intermediates formed along the way would aggregate and be left as off-pathway dead ends without the intervention of a chaperone that resets the folding process .
A current view of protein folding. Each domain of a newly synthesized protein rapidly attains a molten globule state. Subsequent folding occurs more slowly and by multiple pathways, often involving the help of a molecular chaperone.
Molecular chaperones were first identified in bacteria when E. coli mutants that failed to allow bacteriophage lambda to replicate in them were studied. These cells produce slightly altered versions of the chaperone machinery, and as a result they are defective in specific steps in the assembly of the viral proteins. The molecular chaperones are included among the heat-shock proteins , because they are synthesized in dramatically increased amounts after a brief exposure of cells to an elevated temperature . This reflects the operation of a feedback system that responds to any increase in misfolded proteins by boosting the synthesis of the chaperones that help these proteins refold.
I Summary And Perspective
The formation of shell can be described in terms of two major phases: cellular processes of ion transport, protein synthesis, and secretion and a series of physicochemical processes in which crystals of CaCO3 are nucleated, oriented, and grow in intimate association with a secreted organic matrix.
In providing the mineral of shell, Ca2+ and HCO 3 â are first transported across epithelia at the body surface and the mantle epithelium facing the inner shell surface. Movement of these ions is incompletely understood but almost certainly involves active transport of Ca2+. The physicochemical phase takes place in fluid between the outer mantle epithelium and the inner shell surface and on this shell surface. For crystals to be deposited, the following conditions are required concentrations of Ca2+ and CO 3 2- exceeding the solubility product, conditions favoring crystal nucleation, and the elimination of H+ resulting from CaCO3 formation. Following crystal nucleation oriented crystal growth leads to a shell constructed of a variety of crystal patterns. In the process protein secreted by the mantle surrounds the individual crystals and becomes the cement which binds them together as a shell.
Studies indicating involvement of the nervous system and hormonal and neurosecretory changes associated with shell formation will almost certainly be expanded and contribute to the understanding of mechanisms of mineralization and their control.
Laurence Cole, Peter R. Kramer, in, 2016
The First Steps Of In Vivo Folding And Misfolding In The Ribosomes: Cotranslational Versus Posttranslational Processes
A first layer of regulation of protein folding and misfolding in vivo, in which the intracellular milieu composition and PNs begin to operate, appears in the ribosomal synthesis of proteins. In a simple manner, ribosomal protein synthesis consists in three steps: initiation, in which a stable complex at the initiation codon is formed, elongation of the polypeptide chain as the ribosome moves along the actively translated mRNA and the protein remains tethered to the ribosome, and termination of synthesis at the codon stop.18 During ribosomal synthesis, the conformational landscape that is explored by the polypeptide as it grows may differ from that of the protein when is fully synthesized, consequently affecting its folding and misfolding propensities as well as their modulation by the intracellular milieu and PNs.19 The local environment of the ribosome can also significantly modulate protein stability once it has been fully synthesized.21 Therefore, cotranslational and posttranslational processes not present in in vitro studies can be critical to determine protein folding and misfolding in vivo.
C. Shaw, in, 2005
Recommended Reading: Is Paris Jackson Michael’s Biological Kid
There Are Minor Variations In The Standard Genetic Code
As discussed in Chapter 1, the applies to all three major branches of life, providing important evidence for the common ancestry of all life on Earth. Although rare, there are exceptions to this code, and we discuss some of them in this . For example, Candida albicans, the most prevalent human fungal , translates the CUG as serine, whereas nearly all other organisms translate it as leucine. Mitochondria also show several deviations from the standard code. For example, in mammalian mitochondria AUA is translated as methionine, whereas in the of the cell it is translated as isoleucine .
Incorporation of selenocysteine into a growing polypeptide chain. A specialized tRNA is charged with serine by the normal seryl-tRNA synthetase, and the serine is subsequently converted enzymatically to selenocysteine. A specific RNA structure in the
The translational frameshifting that produces the reverse transcriptase and integrase of a retrovirus. The viral reverse transcriptase and integrase are produced by proteolytic processing of a large protein consisting of both
Formation Of Covalent Bonds
Many proteins produced within the cell are secreted outside the cell to function as extracellular proteins. Extracellular proteins are exposed to a wide variety of conditions. In order to stabilize the 3D protein structure, covalent bonds are formed either within the protein or between the different polypeptide chains in the quaternary structure. The most prevalent type is a disulfide bond . A disulfide bond is formed between two cysteine amino acids using their side chain chemical groups containing a Sulphur atom, these chemical groups are known as thiol functional groups. Disulfide bonds act to stabilize the pre-existing structure of the protein. Disulfide bonds are formed in an oxidation reaction between two thiol groups and therefore, need an oxidizing environment to react. As a result, disulfide bonds are typically formed in the oxidizing environment of the endoplasmic reticulum catalyzed by enzymes called protein disulfide isomerases. Disulfide bonds are rarely formed in the cytoplasm as it is a reducing environment.
You May Like: Kuta Software Infinite Geometry Naming Angles
Transfer Rnas Bring Amino Acids To The Ribosome
CAAVal
tRNAs are also RNA polymers. Theyre generally between 75 and 90 RNA nucleotides long. But unlike mRNAs, which are linear, hydrogen bonding between nucleotides within a tRNA causes it to fold up. The resulting shape is often represented as a type of cloverleaf, as shown on left. A more realistic model is shown at right.
On the bottom of the tRNA is an anti-codon: 3 RNA nucleotides that complement the codons in RNA. In other words, for a codon that reads AAA, the corresponding anti-codon would be UUU.
The top of the tRNA has an amino acid binding site.
There are about 45 distinct tRNAs. Each one has a distinct anti-codon. Since there are only 20 amino acids, several of the tRNAs carry the same amino acid .
Protein Synthesis Music Video
Pretty much everything you need to know about protein synthesis is found in my protein synthesis music video. Strap yourself in, because my music partner Max Cowan gives this a heavy metal sound. If youre in a classroom or a computer lab with other students within earshot, please use your headphones/earbuds.
Recommended Reading: Physics Is The Most Basic Science Because
An Mrna Sequence Is Decoded In Sets Of Three Nucleotides
Once an has been produced, by transcription and processing the information present in its sequence is used to synthesize a . Transcription is simple to understand as a means of information transfer: since and are chemically and structurally similar, the DNA can act as a direct for the synthesis of RNA by -pairing. As the term transcription signifies, it is as if a message written out by hand is being converted, say, into a typewritten text. The language itself and the form of the message do not change, and the symbols used are closely related.
In contrast, the conversion of the information in into represents a of the information into another language that uses quite different symbols. Moreover, since there are only four different nucleotides in and twenty different types of amino acids in a protein, this translation cannot be accounted for by a direct one-to-one correspondence between a in RNA and an in protein. The nucleotide sequence of a , through the medium of mRNA, is translated into the amino acid sequence of a protein by rules that are known as the . This code was deciphered in the early 1960s.
The genetic code. The standard one-letter abbreviation for each amino acid is presented below its three-letter abbreviation . By convention, codons are always
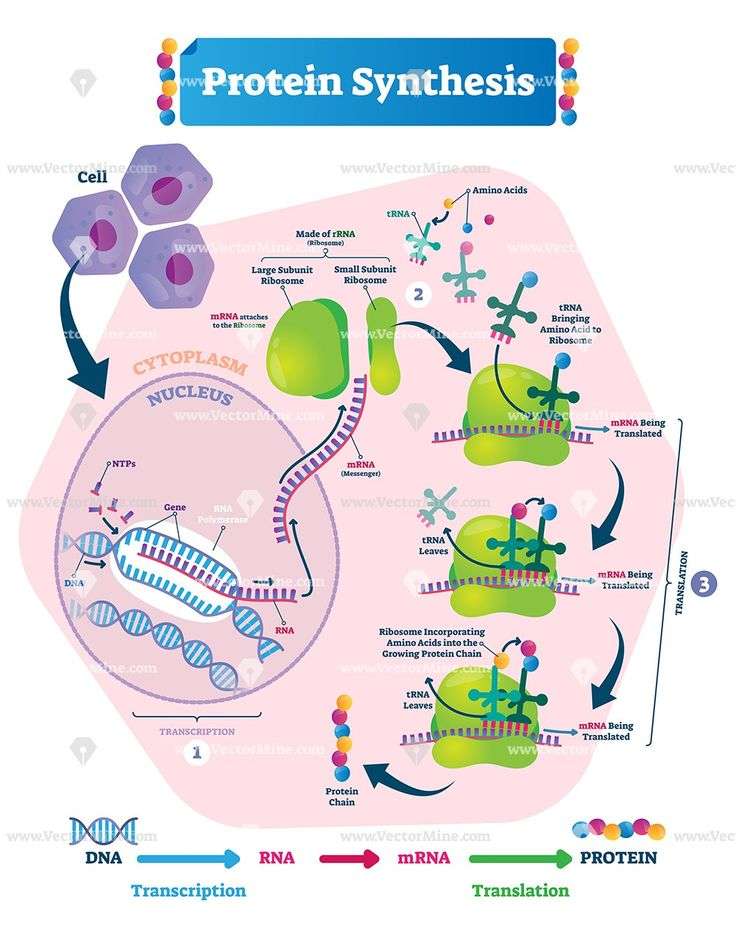
A Short Explanation Of The Fascinating Process Of Protein Synthesis
Protein synthesis refers to the construction of proteins by the living cells. Comprising two primary parts , the process of protein synthesis involves ribonucleic acids , deoxyribonucleic acid , enzymes, and ribosomes.
Like it? Share it!
Protein synthesis refers to the construction of proteins by the living cells. Comprising two primary parts , the process of protein synthesis involves ribonucleic acids , deoxyribonucleic acid , enzymes, and ribosomes.
Proteins are important organic compounds present in living organisms. They are essential in almost all cell functions. Specific proteins are involved with particular functions. Proteins are made up of long chains of amino acids, which are either arranged in a linear pattern, or folded to form a complex structure.
Based on the structural complexity, structure of proteins is classified into four types primary, secondary, tertiary, and quaternary. Also, the types of amino acids play a crucial role in determining the expression of genes in this process.
Protein synthesis is a biological procedure performed by living cells to manufacture proteins in a step-by-step manner. Many times, it is used to denote translation, which otherwise is a primary part in the protein synthesis process. When studied in detail, the synthesis process is very complex. The process itself begins with production of different amino acids, out of which some are derived from food sources.
Recommended Reading: Core Connections Algebra Chapter 11 Answers
How Does A Gene Direct The Synthesis Of A Protein
DNA holds the code for a gene in its sense strand, which runs 5 ‘to 3’. This base sequence is transferred onto an mRNA strand during transcription, using the antisense strand. At the ribosomes, tRNA, which contains a complementary anticodon, delivers the respective amino acid to the site. This means the building of the polypeptide chain is
purely informed by the gene.
Protein Folding Modification And Targeting
During and after translation, individual amino acids may be chemically modified, signal sequences may be appended, and the new protein folds into a distinct three-dimensional structure as a result of intramolecular interactions. A signal sequence is a short tail of amino acids that directs a protein to a specific cellular compartment. These sequences at the amino end or the carboxyl end of the protein can be thought of as the proteins train ticket to its ultimate destination. Other cellular factors recognize each signal sequence and help transport the protein from the cytoplasm to its correct compartment. For instance, a specific sequence at the amino terminus will direct a protein to the mitochondria or chloroplasts . Once the protein reaches its cellular destination, the signal sequence is usually clipped off.
Many proteins fold spontaneously, but some proteins require helper molecules, called chaperones, to prevent them from aggregating during the complicated process of folding. Even if a protein is properly specified by its corresponding mRNA, it could take on a completely dysfunctional shape if abnormal temperature or pH conditions prevent it from folding correctly.
Read Also: Geometry Chapter 10 Test Form B Answers
Many Proteins Are Controlled By Regulated Destruction
One function of intracellular proteolytic mechanisms is to recognize and eliminate misfolded or otherwise abnormal proteins, as just described. Yet another function of these proteolytic pathways is to confer short half-lives on specific normal proteins whose concentrations must change promptly with alterations in the state of a cell. Some of these short-lived proteins are degraded rapidly at all times, while many others are conditionally short-lived, that is, they are metabolically stable under some conditions, but become unstable upon a change in the cell’s state. For example, mitotic cyclins are long-lived throughout the cell cycle until their sudden degradation at the end of , as explained in Chapter 17.
How is such a regulated destruction of a controlled? A variety of mechanisms are known, as illustrated through specific examples later in this book. In one general class of mechanism , the activity of a is turned on either by E3 or by an allosteric transition in an E3 protein caused by its binding to a specific small or large . For example, the is a multisubunit ubiquitin ligase that is activated by a cell-cycle-timed addition at . The activated APC then causes the degradation of mitotic cyclins and several other regulators of the -anaphase transition .
What Are The Translation Steps In Protein Synthesis
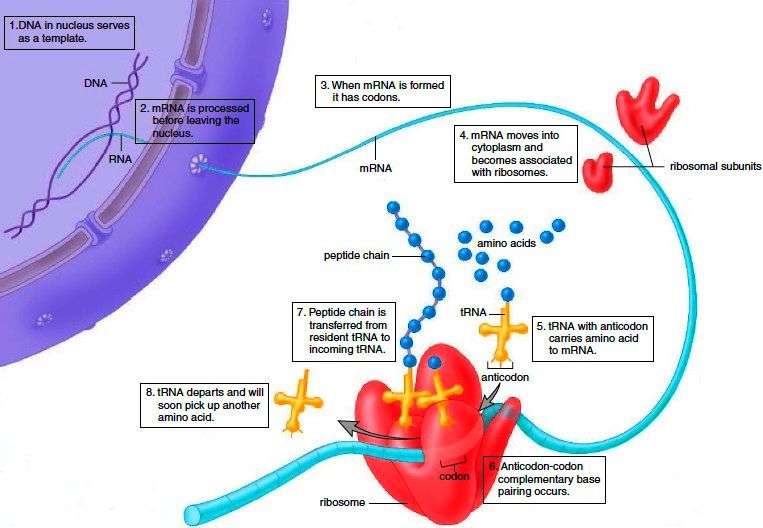
Ribosomes are organelles responsible for the translation of mRNA, a term that describes the ‘reading’ of the genetic code. These organelles, which are made of ribosomal RNA and proteins, hold the mRNA in place throughout this step. The ‘reading’ of the mRNA begins when the start codon, AUG, is detected.
First, we’ll need to know about transfer RNA . These clover-shaped polynucleotides contain two important features:
- An anticodon, which will bind to its complementary codon on the mRNA.
- An attachment site for an amino acid.
Ribosomes can harbour a maximum of two tRNA molecules at a time. Think of tRNAs as the vehicles delivering the correct amino acids to the ribosomes.
Below are the steps for translation:
- The mRNA binds to the small subunit of a ribosome at the start codon, AUG.
- A tRNA with a complementary anticodon, UAC, binds to the mRNA codon, carrying with it the corresponding amino acid, methionine.
- Another tRNA with a complementary anticodon for the next mRNA codon binds. This allows the two amino acids to come close.
- The enzyme, peptidyl transferase, catalyses the formation of a peptide bond between these two amino acids. This uses ATP.
- The ribosome travels along the mRNA and releases the first bound tRNA.
- This process repeats until a stop codon is reached. At this point, the polypeptide will be complete.
Figure 3. Ribosome mRNA translation Source: Wikimedia Commons Public Domain
Also Check: Practice And Homework Lesson 4.5
The Process Of Translation: A Detailed Look
This chapter began with an overview of translation and then described in more detail what is happing in the ribosome and how the amino acid chain builds. Now watch the following video, which is an in-depth version of the first video of this chapter, now incorporating aspects described throughout this chapter.
For closed captioning or to view the full transcript, click on the YouTube link in the video and view the video on YouTube.
The animations above focused on what happens at a single ribosome. In reality, though, an mRNA molecule may have several ribosomes attached to it at once, making multiple copies of the protein. They all begin at the first codon, but as one starts moving down the mRNA, another can attach therefore, they all make the same protein.
Previous/next navigation
Exposed Hydrophobic Regions Provide Critical Signals For Protein Quality Control
If radioactive amino acids are added to cells for a brief period, the newly synthesized proteins can be followed as they mature into their final functional form. It is this type of experiment that shows that the hsp70 proteins act first, beginning when a is still being synthesized on a , and that the hsp60-like proteins are called into play only later to help in folding completed proteins. However, the same experiments reveal that only a subset of the newly synthesized proteins becomes involved: perhaps 20% of all proteins with the hsp70 and 10% with the hsp60-like molecular chaperones. How are these proteins selected for this ATP-catalyzed refolding?
Before answering, we need to pause to consider the post-translational fate of proteins more broadly. A that has a sizable exposed patch of hydrophobic amino acids on its surface is usually abnormal: it has either failed to fold correctly after leaving the , suffered an accident that partly unfolded it at a later time, or failed to find its normal partner in a larger protein . Such a protein is not merely useless to the cell, it can be dangerous. Many proteins with an abnormally exposed hydrophobic region can form large aggregates, precipitating out of solution. We shall see that, in rare cases, such aggregates do form and cause severe human diseases. But in the vast majority of cells, powerful protein quality control mechanisms prevent such disasters.
You May Like: Ccl4 Dot Structure
Reproduction The Genome And Gene Expression
The differences between sexual and asexual reproduction, the structure of DNA and its role in making proteins, mutations and their effects and how characteristics are inherited.
Greg Foot explains how the structure of DNA affects the proteins made in DNA synthesis
The DNA code for the protein remains in the nucleus, but a copy, called mRNA, moves from the nucleus to the ribosomes where proteins are synthesised in the cytoplasm. The protein produced depends on the template used, and if this sequence changes a different protein will be made.
Carrier molecules bring specific amino acids to add to the growing protein in the correct order. There are only about 20 different naturally-occurring amino acids.
Each protein molecule has hundreds, or even thousands, of amino acids joined together in a unique sequence. It is then folded into the correct unique shape. This is very important, as it allows the protein to do its job. Some proteins are enzymes, others are hormones and others form structures within the body, such as collagen. Each of these proteins needs a different shape.
Cells express their genes by converting the genetic message into protein. This process of protein synthesis occurs in two stages – transcription and translation.