S Of Drawing Lewis Structure Of So32
Following steps are required to draw the SO32- lewis structure and they are explained in detail in this tutorial.
Drawing correct lewis structure is important to draw resonance structures correctly
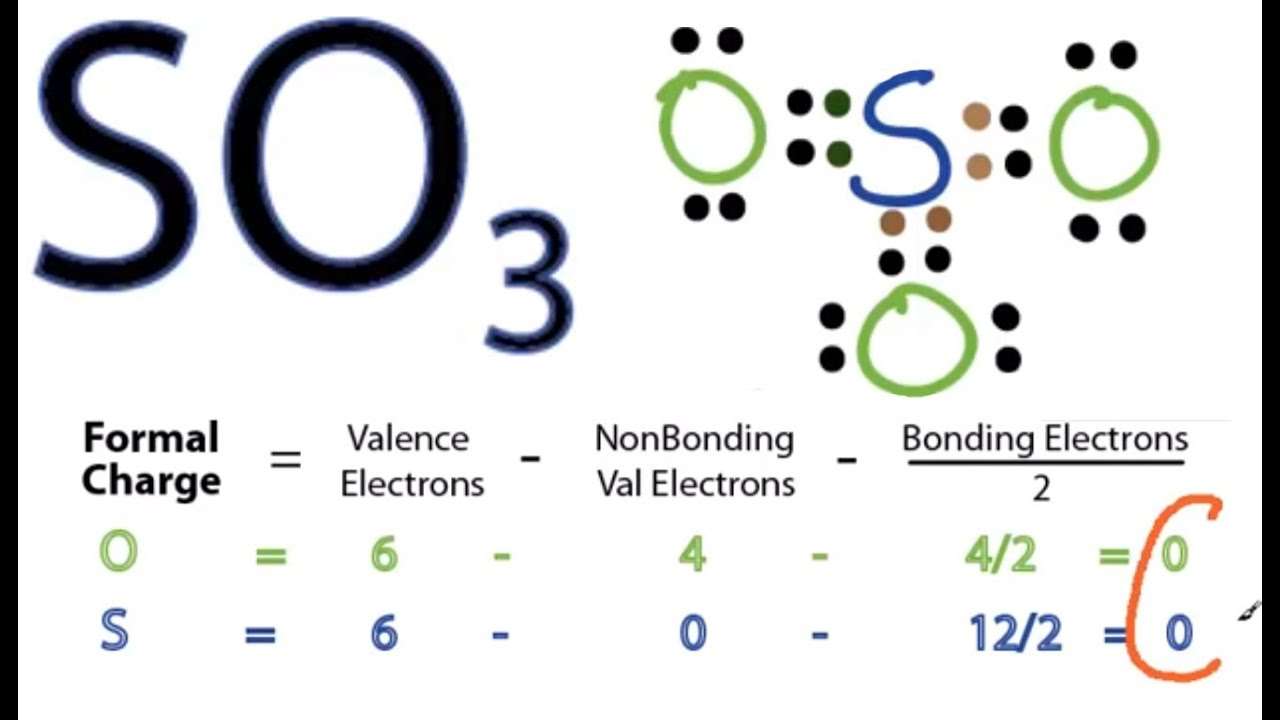
Calculating Formal Charge On The Sulfur Of So3 Molecule:
The formal charge on the SO3 moleculeâs sulfur central atom often corresponds to the actual charge on that sulfur central atom. In the following computation, the formal charge will be calculated on the central sulfur atom of the SO3 Lewis dot structure.
To calculate the formal charge on the central sulfur atom of the SO3 molecule by using the following formula:
The formal charge on the sulfur atom of SO3 molecule= â L.E â 1/2)
V.E = Valence electron in a sulfur atom of SO3 molecule
L.E = Lone pairs of an electron in the sulfur atom of the SO3 molecule.
B.E = Bond pair electron in S atom of SO3 molecule
calculation of formal charge on sulfur atom in SO3 molecule
The sulfur core atom of the SO3 molecule has six valence electrons, zero lone pair of electrons, and 12 bonding pairing valence electrons. Put these values for the sulfur atom in the formula above.
Formal charge on sulfur atom of SO3 molecule = ) =0
In the Lewis structure of SO3, the formal charge on the central sulfur atom is zero.
Check The Stability And Minimize Charges On Atoms By Converting Lone Pairs To Bonds
- Oxygen atoms should hold negative charges because electronegativity of oxygen is higher than sulfur. Otherwise, we can say, ability of holding negative charges is great in oxygen atoms than sulfur atoms.
- The drawn structure is not a stable one because all atoms have a charge.
- Now, we should try to minimize charges by converting lone pair or pairs to bonds. So convert one lone pair of one oxygen atom to make a S-O bond.
- Now there is a double bond between sulfur atom and one oxygen atom. Now, there are two single bonds between sulfur atom and other two oxygen atoms.
In new structure, charges of atoms are reduced than previous structure. Now there is no charge on sulfur atom. Also, only two oxygen atoms have -1 negative charges. Now you understand this structure of SO32- is more stable than previous structure. So, this structure has more chance to be the lewis structure of SO32- ion.
Read Also: Algebra With Pizzazz Books Never Written
The Influence Of Thermal Excitation
Since the motions of the atoms in a molecule are determined by quantum mechanics, “motion” must be defined in a quantum mechanical way. The overall quantum mechanical motions translation and rotation hardly change the geometry of the molecule. In addition to translation and rotation, a third type of motion is molecular vibration, which corresponds to internal motions of the atoms such as bond stretching and bond angle variation. The molecular vibrations are harmonic , and the atoms oscillate about their equilibrium positions, even at the absolute zero of temperature. At absolute zero all atoms are in their vibrational ground state and show zero point quantum mechanical motion, so that the wavefunction of a single vibrational mode is not a sharp peak, but an exponential of finite width . At higher temperatures the vibrational modes may be thermally excited , but they oscillate still around the recognizable geometry of the molecule.
To get a feeling for the probability that the vibration of molecule may be thermally excited,we inspect the Boltzmann factorβ â¡ exp, where ÎE is the excitation energy of the vibrational mode, k the Boltzmann constant and T the absolute temperature. At 298 K , typical values for the Boltzmann factor β are:
- β = 0.0890 for ÎE = 0500 cmâ1
- β = 0.0080 for ÎE = 1000 cmâ1
- β = 0.0007 for ÎE = 1500 cmâ1.
What Is The Electron Geometry Of So3

Sulfur trioxide has a trigonal planar electron geometry, according to David Roth of Tutoring & Homework Help.SO3 has a central sulfur atom and three surrounding oxygens, with a total of 24 valence electrons. Two oxygens form single bonds with sulfur, while one forms a double bond.
The central sulfur atom of SO3 has an oxidation state of +6 and a formal charge of +2. In an SO3 compound, three oxygen atoms are positioned at the corners of a triangle, all in one three-dimensional plane. The bond angles between all oxygens is 120 degrees. SO3 is centrosemetric and has no electric dipole.
Also Check: Glencoe Mcgraw Hill Geometry Workbook Answers
Sulfur Trioxide Molecular Geometry
Being an intelligent and well-practiced human being, you must know what is molecular geometry, but let me revise it for the all young students out there. Molecular geometry is the three-dimensional structure of the atoms which helps in the constitution of a molecule. It can determine reactivity, polarity, color, attraction, biological activity, etc.
SO3 includes two components mainly Sulfur and Oxygen. There are one sulfur atom and three oxygen atoms which are spread out as far away as they can! Atoms of oxygen are surrounded by electrons. These electrons are negative and repel each other.
You can also remember it by AXN. Where
- A stands for Sulfur, which is central atom
- X stands for No. of atoms bonded with central sulfur
- N stands for any nonbonding electron pairs
In this formula of SO3, we dont have any non-bonding electron, and that is why we dont bother about N. Moreover, as there are three oxygen, it will be X3.
That means we have AX3 for the SO3 molecule. The bond angle of SO3 is 120 degrees.
Resonance Structures Of So32
Change the location of double bond and lone pairs of molecule to draw resonance structures of SO32- ion. Three stable resonance structures can be drawn of SO32- ion.
Questions
In sulfite and carbonate ions, are there similar number of lone pairs on all oxygen atoms?
Yes. Each sulfite and carbonate ions contain three oxygen atoms. By In both Lewis structures, there are eight lone pairs on all oxygen atoms.
how many electron groups are around the central atom? so32- ion
Central atom of SO32- ion is sulfur. Around sulfur atom, there are four bonds and a single lone pair in the lewis structure of SO32- ion. Therefore, five electron groups are around the central atom of SO32- ion.
Is there are charges on sulfur atom in sulfite ion lewis structure?
There are no charges in sulfur atom. Only negative charges exists in two oxygen atoms.
Are there pi bonds in sulfite Lewis structure?
There is only one pi bond and it is located at between one pxygen atom and sulfur atom.
Is lewis structure for so32- is different from lewis structure for so42-
Yes. They are different. In SO42- lewis structure, there are four oxygen atoms around the sulfur atom. Therefore number of valence electrons pairs are different in two ions.
You May Like: How To Calculate Net Force With Angles
Why So3 Forms Double Bonds
Because of equal formal charge distribution throughout the atom, double covalent bonds form in SO3.
It is determined by the number of electrons an atom brought number of lone pair of electrons half the number of electrons in bond formation.
So, for oxygen, it is 6-6-1 = -1
And for sulfur, it is 6-0-3 = +3.
Now, the formal charge needs to be neutralized to achieve a stable condition, so having +3 at centre and -1 at the ends cannot happen. It is the reason why three double covalent bonds are formed in SO3.
Overview: So3 Lewis Structure
The central atom is sulfur, which is bordered on three terminals with oxygen atoms, and zero lone pair on the central in the trigonal planar geometry. Sulfur has six outermost valence electrons, indicating that it possesses six electrons in its outermost shell, whereas oxygen also has six valence electrons in its outermost shell. To complete the octet of the sulfur and oxygen atoms requires two valence electrons on each of their outermost shell.
Three oxygen atoms establish covalent connections with the central sulfur atom as a result, leaving the sulfur atom with zero lone pair. There are zero lone pair of electron on the sulfur central atom that resists the bond pairs of the three S-O bonds. According to VSEPR theory, the double S-O bond pairs polarity lead the SO3 molecule to take on the trigonal planar geometry structure.
The SO3 moleculeâs three S-O bonds are arranged in symmetrical polarity order around the trigonal planar molecular geometry, giving rise to the SO3 molecular shape. The SO3 molecule has a trigonal planar molecular geometry because there is electrical repulsion between the lone pairs of electrons in oxygen and three double bond pairs of the SO3 molecule.
Read Also: Michael Jackson Kids Biological
Molecular Geometry Of Sulphur Trioxide
The three-dimensional structure of the atoms that aids in forming a molecule is known as molecular geometry. It can tell you about reactivity, polarity, colour, attraction, and biological activity, among other things.
In Sulphur Trioxide, one sulphur atom and three oxygen atoms are stretched as far apart as possible! Electrons encircle oxygen atoms. Negative electrons repel negative electrons.
AXN is another way to remember the molecular geometry of SO3.
- Sulphur, the centre atom, is represented by the letter A.
- The letter X denotes the number of atoms bound to central sulphur.
- Any nonbonding electron pairs are denoted by the letter N.
- Because there are no nonbonding electrons in this SO3 formula, we dont need to worry about N. Furthermore, because there are three oxygen atoms, the result will be X3.
As a result, the SO3 molecule has AX3. SO3 has a bond angle of 120 degrees.
Stability Of Structure And Minimize Charges On Atoms By Converting Lone Pairs To Bonds
When there are positive and negative charges on lot of atoms or higher charges on atoms in an ion or molecule, that structure is not stable one. Therefore, We should try to reduce charges on atoms if it is a possible. In the above structure, there are charges on oxygen atoms and sulfur atom. Now, we are going to reduce charges on these atoms as below.
- Step 1: Now, we should try to minimize charges by converting a lone pair or pairs to a bond. So convert a lone pair on a oxygen atom to make a new S-O bond with sulfur atom as the following figure.
- Now there is a double bond between one oxygen atom and sulfur atom. You can see, charges are reduced now in the new structure.
- Step 2: Again, we can convert a lone pair on another oxygen atom to make a S-O bond. Now, another double bond is formed. In the new structure, there are two double bonds.
- Step 3: Because, there are still charges on atoms, we can try to convert a lone pair to a bond. So, convert a lone pair on other oxygen atom to make a bond with sulfur atom. With that, there are no charges on sulfur and oxygen atoms and that structure is stable than other previous structures.
- Therfore, that structure should be the lewis structure of SO2
Questions
Also Check: Unit 1 Test Study Guide Geometry Basics Gina Wilson
What Is The Shape Of Nh3
trigonal pyramidal shapeThe ammonia molecule has a trigonal pyramidal shape with the three hydrogen atoms and an unshared pair of electrons attached to the nitrogen atom.
Is SO3 trigonal planar domain geometry?
The molecular geometry of SO3 is trigonal planar with symmetric charge distribution on the central atom.
What Does So3 Stand For
SO3 stands for Sulfur Trioxide. This is one of the most pollutant chemical compounds in the gaseous form. It is also a primary agent in the acid rain.
What is the molecular shape of sulfur trioxide?
Moreover, through the valence shell electron pair repulsion theory, the structure of sulfur trioxide is found to be bent shaped or trigonal pyramidal or trigonal planar, where the bond angle is 120°.
Recommended Reading: What Is Elastic Force In Physics
Calculating Formal Charge On The Oxygen Atom Of So3 Molecule:
The formal charge on the SO3 moleculeâs oxygen terminal atoms often corresponds to the actual charge on that oxygen terminal atoms. In the following computation, the formal charge will be calculated on the terminal oxygen atom of the SO3 Lewis dot structure.
To calculate the formal charge on the terminal oxygen atom of the SO3 molecule by using the following formula:
The formal charge on the oxygen atom of SO3 molecule= â L.E â 1/2)
V.E = Valence electron in a oxygen atom of SO3 molecule
L.E = Lone pairs of an electron in the oxygen atom of the SO3 molecule.
B.E = Bond pair electron in O atom of SO3 molecule
calculation of formal charge on oxygen atom in SO3 molecule
The oxygen terminal atoms of the SO3 molecule have six valence electrons, two lone pairs of electrons, and four bonding pairing valence electrons. Put these values for the oxygen atom in the formula above.
Formal charge on oxygen atom of SO3 molecule = ) =0
In the Lewis structure of SO3, the formal charge on the terminal oxygen atom is zero.
Calculate The Number Of Molecular Hybridizations Of The So3 Molecule
What is SO3 hybridization? This is a very fundamental question in the field of molecular chemistry. All the molecules are made of atoms. In chemistry, atoms are the fundamental particles. There are four different types of orbitals in chemistry. They are named s, p, d, and f orbitals.
The entire periodic table arrangement is based on these orbital theories. Atoms in the periodic table are classified as follows:
s- block elements
f-block elements
Atoms are classified in the periodic table
SO3 molecule is made of one sulfur, three oxygen atoms. The oxygen and sulfur atoms have s and p orbitals. Oxygen comes as the first element from the oxygen family in the periodic table. The sulfur atom also belongs to the same family group. But it falls as the second element in the periodic table.
When these atoms combine to form the SO3 molecule, its atomic orbitals are mixed and form unique molecular orbitals due to hybridization.
How do you find the SO3 moleculeâs hybridization? We must now determine the molecular hybridization number of SO3.
The formula of SO3 molecular hybridization is as follows:
No. Hyb of SO3= N.A + L.P
No. Hy of SO3 = the number of hybridizations of SO3
Number of S-O bonds = N.A
Lone pair on the central sulfur atom = L.P
Calculation for hybridization number for SO3 molecule
No. Hyb of SO3= 3+0=3
Also Check: Paris Jackson Mark Lester
Discover And Learn What Students Are Asking
Is So3 Polar Or Nonpolar
The polarity of the molecule results from the non-symmetrical sharing of the valence electron, creating a region of unequal charges in the molecule.
Based on the Linus Pauling scale of electronegativity, the electronegativity of sulfur is 2.4 while the electronegativity of oxygen is 3.44. Because of the trigonal planar form of sulfur trioxide, SO3 is nonpolar. Because sulfur and oxygen have different electronegativity, polarity develops in the S-O bond. However, because the three S-O bonds are at a 120-degree angle to each other, the overall polarity is canceled out, resulting in the formation of SO3 as a nonpolar molecule. Therefore, SO3 is a nonpolar compound.
Electronegativity of SO3
As we know, Electronegativity is the dimensionless property of atoms in compounds to attract the shared pairs of electrons towards themselves. Below is the property of how electronegativity affects the polarity of compounds.
- If the electronegativity difference is greater than 1.7, the bond will have an ionic character.
- If the electronegativity difference is between 0.4 and 1.7, the bond will have a polar covalent character.
- If the electronegativity difference is less than 0.4, the bond will have a nonpolar covalent character.
Read Also: The Shape Of Ccl4 Is
Center Atom And Sketch Of Ethene Molecule
There are several requirements to be the center atom in a molecule. We have to think whether center atom is sulfur or oxygen. Having a high valence is a main requirement to be a center atom. For SO3 molecule, sulfur has the highest valence than and oxygen. Therfore, sulfur is the center atom in SO3. Structure of atoms of SO3 is figured below.
Trigonal Planar Molecular Geometry
| Trigonal planar molecular geometry |
|---|
| 0 |
In chemistry, trigonal planar is a molecular geometry model with one atom at the center and three atoms at the corners of an equilateral triangle, called peripheral atoms, all in one plane. In an ideal trigonal planar species, all three ligands are identical and all bond angles are 120°. Such species belong to the point groupD3h. Molecules where the three ligands are not identical, such as H2CO, deviate from this idealized geometry. Examples of molecules with trigonal planar geometry include boron trifluoride , formaldehyde , phosgene , and sulfur trioxide . Some ions with trigonal planar geometry include nitrate , and guanidinium +3). In organic chemistry, planar, three-connected carbon centers that are trigonal planar are often described as having sp2 hybridization.
Nitrogen inversion is the distortion of pyramidal amines through a transition state that is trigonal planar.
Pyramidalization is a distortion of this molecular shape towards a tetrahedral molecular geometry. One way to observe this distortion is in pyramidal alkenes.
Read Also: Half Life Equations Chemistry