What Is Ionic Bond Definition
A chemical bond is a lasting attraction between these atoms, ions or molecules that enables the formation of chemical compounds. The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic bonds or through the sharing of electrons as in covalent bonds. Therefore, the electromagnetic force plays a major role in determining the internal properties of most objects encountered in daily life.
What Are The Properties Of Ionic Or Electrovalent Compounds
1. Ionic Compounds are Hard Solids.
This is because their constituent particles are ions which are held by the strong electrostatic force of attraction and hence they cannot be separated easily.
2. Ionic Compounds Have A High Melting Point and High Boiling Point.
They are non-volatile solids. As in these compounds, ions are held by the strong electrostatic force of attraction, so a large amount of energy is required to overcome these forces of attraction between the ions.
3. Ionic Compounds Do Not Conduct Electricity in Their Solid-State.
However, they can conduct electricity in their fused, molten and in their aqueous solution. In solid-state, they do not conduct electricity as the ions are not free but held by the strong electrostatic force. But infused or molten state, these forces of attraction get weakened and thus the ions become free to conduct electricity. In aqueous solution, the high dielectric constant overcomes the strong electrostatic force of attraction, thus making the ions free to conduct electric current.
4.Ionic Compounds Act as Strong Electrolytes.
As on dissolving in water, ionic compounds allow the passage of electric current through them due to the presence of free ions.
5. Ionic Compounds are Soluble in Water but Insoluble in Organic Solvents like Benzene and Phenol.
6. On passing an electric current through fused, molten and aqueous solution of electrovalent compounds, the ions dissociate and migrate towards electrodes.
Formation Of Ionic Bond
An ionic bond can be formed after two or more atoms loss or gain electrons to form an ion. Ionic bonds occur between metals, losing electrons, and nonmetals, gaining electrons. Ions with opposite charges will attract one another creating an ionic bond. Such bonds are stronger than hydrogen bonds, but similar in strength to covalent bonds.
Eric Stauffer, … Reta Newman, in, 2008
Read Also: Difference Between Human Geography And Physical Geography
What Is An Ionic Bond
The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond.
A chemical bond is formed between two atoms by the complete transfer of one or more electrons from one atom to the other as a result of which the atoms attain their nearest inert gas configuration.
There are primarily three ways in which two atoms combine to lose energy and to become stable. One of the ways is by donating or accepting electrons to complete their octet configuration. The bond formed by this kind of combination is known as an ionic bond or electrovalent bond. This kind of bond is formed when one atom gains electrons while the other atom loses electrons from its outermost level or orbit.
Ionic Vs Molecular Compounds
Ionic compounds are pure substances formed from chemically bonded ions. Ionic bonds form ionic compounds. Covalent bonds form molecular compounds.
Most atoms join by covalent bonding, in which shared electrons form directional bonds. These are molecules.
Ionic compounds are electrically neutral and can form from just a few atoms or from large numbers of atoms . Two-element ionic compounds and polyatomic ionic compounds are equally common.
Polyatomic ions are ions comprised of more than one atom. A good example is the ammonium ion made up of one nitrogen atom and four hydrogen atoms.
Ions that are negatively charged are called anions, pronounced an-ions. Common anions are non-metals. Ions with net positive charges are called cations, pronounced cat-ions. Metals are commonly cations.
You May Like: Span In Linear Algebra
Introduction Of Ionic Bond
Ionic bond is a kind of chemical bond which involves an electrostatic attraction between two oppositely charged ions because of the complete transfer of valence electrons between them. As for example: metals such as sodium losses electrons to to become positive ion, whereas non-metal such as chlorine accepts electrons to become a negative ion. The metal that gives electrons is called donor and the non-metal that accepts electrons is called acceptor.
Common Questions About Ionic Bonding In Chemistry
Q: What is ionic bonding?
In ionic bonding, electrons are transferred from one atom to another, resulting in the formation of positive and negative ions. Its the attraction created between positive and negative ions that creates a compound.
Q: What are some examples of ionic bonding?
There are many examples of ionic bonding in nature. Table salt is an excellent example of an ionic bond between sodium and chlorine. Silicon and oxygen also bond with a strong ionic bond to form quartz. It should be noted that the beach sand is made of quartz.
Q: What are the properties of ionic bonding?
Ionic bonding has three unique properties. These features include high resistance, electrical insulation, and transparency.
Also Check: Edgenuity Unit Test Answers
Electronegativity And Ionic Bonding
- An Ionic bond is the bond formed by the complete transfer of valence electron to attain stability.
- This type of bonding leads to the formation of two oppositely charged ions positive ion known as cations and negative ions are known as anions.
- The presence of two oppositely charged ions results in a strong attractive force between them. This force is an ionic or electrovalent bond.
- Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity.
- The compound formed by the electrostatic attraction of positive and negative ions is called an ionic compound.
Strength Of The Bonding
For a solid crystalline ionic compound the enthalpy change in forming the solid from gaseous ions is termed the lattice energy.The experimental value for the lattice energy can be determined using the BornHaber cycle. It can also be calculated using the BornLandé equation as the sum of the electrostatic potential energy, calculated by summing interactions between cations and anions, and a short-range repulsive potential energy term. The electrostatic potential can be expressed in terms of the interionic separation and a constant that takes account of the geometry of the crystal. The further away from the nucleus the weaker the shield. The Born-Landé equation gives a reasonable fit to the lattice energy of, e.g., sodium chloride, where the calculated value is 756 kJ/mol, which compares to 787 kJ/mol using the BornHaber cycle. In aqueous solution the binding strength can be described by the Bjerrum or Fuoss equation as function of the ion charges, rather independent of the nature of the ions such as polarizability or size The strength of salt bridges is most often evaluated by measurements of equilibria between molecules containing cationic and anionic sites, most often in solution. Equilibrium constants in water indicate additive free energy contributions for each salt bridge. Another method for the identification of hydrogen bonds also in complicated molecules is crystallography, sometimes also NMR-spectroscopy.
Don’t Miss: What Is Span Linear Algebra
What Kind Of Force Is Present In Ionic Bonds
Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. For both atoms involved, this exchange results in a more stable noble gas electrical state. The attractive electrostatic interactions between two ions of opposite charge form an ionic bond.
What Is An Ionic Compound
An ionic compound usually consists of a metal and a non-metal. When lots of ions come together, they form large structures called ionic lattices. Ions in an ionic lattice arrange themselves in a regular, 3D shape with oppositely charged ions next to one another. This structure is also sometimes referred to as a crystal lattice.
Also Check: Geometry Chapter 10 Test Form B Answers
Ionic Bond Vs Covalent Bond
| Ionic Bond | Covalent Bond |
| The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. | The covalent bond is a bond formed when two atoms share one or more electron pairs. Each atom contributes an equal number of electrons towards the bond formation. |
| If the difference of ionization potential between the two atoms is more ionic compounds are formed. | Atoms with higher ionization potential are unable to lose their valence electrons and hence prefer to form covalent bonds by sharing of electrons. |
| Atoms with greater electronegativity difference lead to the formation of an ionic bond. | If the electronegativities of the combining atoms do not differ much then the bond formed between them is likely to be covalent. |
| Example: NaCl | Example: HCl |
Differences Between Compounds With Covalent And Ionic Bonds
The definition of an ionic compound, is a chemical compound composed of ions held together by electrostatic forces basically held together by ionic bonds. They are formed by neatly packed ions of opposite charge. The compound is neutral, but it consists of positively and negatively charged cations and anions. Lets look at some differences between ionic and covalent bonds and compounds.
We hope that gives you a better sense of ionic vs covalent.
Recommended Reading: Geometry Dash Toe 2
Example: Sodium Chloride Formation By Ionic Bond
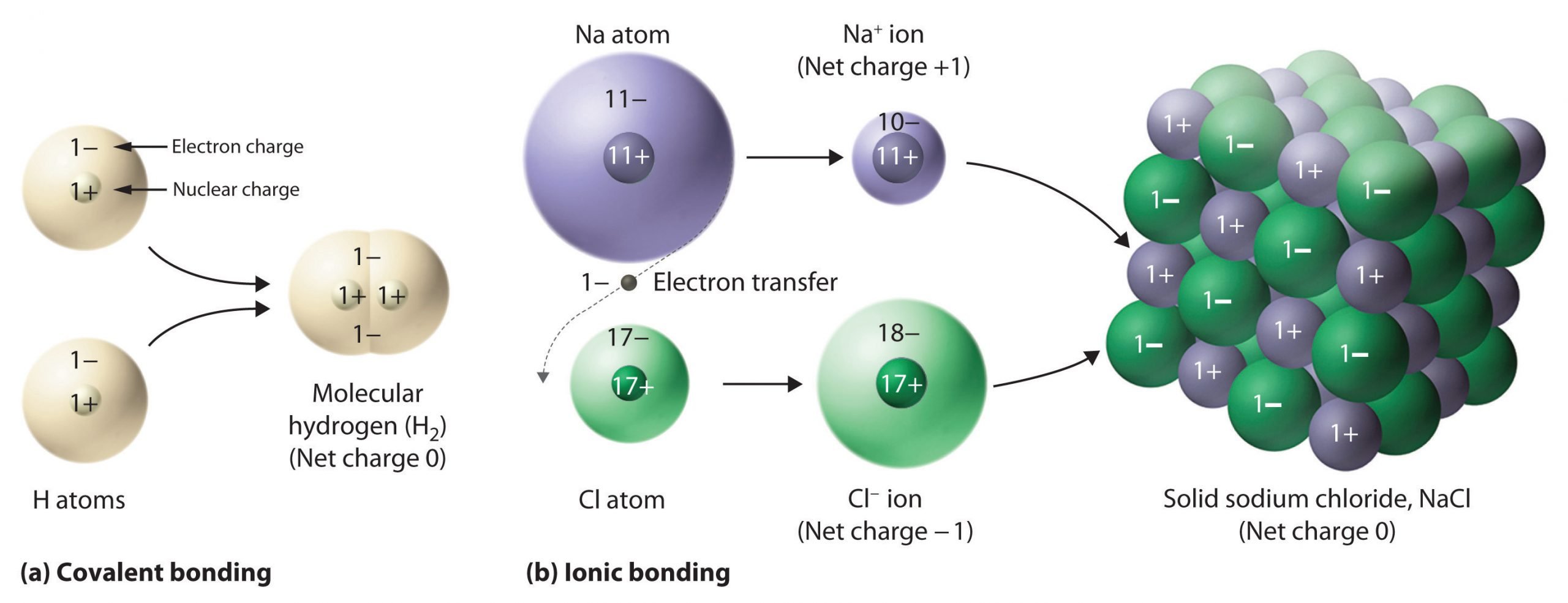
There is only one electron in the outermost shell of Sodium atom and if it loses that electron from its outermost shell i.e. the M shell then the L shell becomes the outermost shell and it has a stable octet. There are eleven protons in the nucleus of this atom but the number of electrons has become ten, this gives the sodium atom a positive charge and creates a sodium cation Na+. Whereas, on the other hand, there are seven atoms in the outermost shell of the Chlorine atom then it needs one more electron to complete its octet. If Sodium and Chlorine reacted then the electron lost by sodium would be taken by Chlorine. After this process, the chlorine atom would have a negative charge, because it would have seventeen protons in its nucleus and there would be eighteen electrons in its K, L and M shells. This gives the Chlorine atom a negative charge. Hence, the negatively charged Chlorine and positively charged Sodium creates an Ionic bond or an Electrovalent compound called Sodium Chloride, commonly known as Common Salt.
Image will be uploaded soon
Properties Of Ionic Compounds
Ionic compounds form crystalline structures, called crystal lattices. Common table salt N
- O 4
These are built up in repeated patterns of unit cells, as contrasted with molecules. One piece of an ionic compound that maintains all the entire compound’s characteristics is called a unit.
In comparison, one piece of a covalently bonded compound that maintains all its entire compound characteristics is called a molecule.
Ionic compounds dissolve in polar solvents such as water. Water can dissolve ions and polar molecules. Non-polar solvents do not dissolve ionic compounds.
Other physical properties of ionic compounds:
- High melting points
- They are generally hard and brittle
- They are electrically neutral
- They form efficient insulators
- High enthalpies of fusion and vaporization
You May Like: Theory Of Everything 2 Music
How Are Ionic Bonds Formed
- Ionic bonds are formed between atoms that have an electronegativity difference of 1.7 or higher.
- When such atoms come closer, the difference in electronegativity causes an unequal sharing of electrons so that one atom completely loses an electron while the other accepts the electron.
- Ionic bond formation occurs through redox reaction when the atom with low ionization energy gives one or more electrons to reach a stable electron configuration. The resulting chemical units are termed cations.
- The atom of another element with high electron affinity accepts the electron from the other atom to receive a stable electron configuration. The resulting chemical species are termed anions.
- The strength of ionic bonds depends on the electrovalency of the atoms as the bonds are formed in order to achieve the electronic configuration of noble gases in different blocks.
- During the formation of ionic bonds, strict ratios are observed between anions and cations as the ionic compounds follow the rules of stoichiometry.
- The formation of ionic bonds occurs only if the overall change in the energy of the system is favourable.
- The formation of cations or the removal of electrons is an endothermic process which raises the energy of the system. The energy, however, is lowered during the subsequent attraction between the ions and the acceptance of electrons by the anions.
Comparison With Covalent Bonding
In ionic bonding, the atoms are bound by attraction of oppositely charged ions, whereas, in covalent bonding, atoms are bound by sharing electrons to attain stable electron configurations. In covalent bonding, the molecular geometry around each atom is determined by valence shell electron pair repulsion VSEPR rules, whereas, in ionic materials, the geometry follows maximum packing rules. One could say that covalent bonding is more directional in the sense that the energy penalty for not adhering to the optimum bond angles is large, whereas ionic bonding has no such penalty. There are no shared electron pairs to repel each other, the ions should simply be packed as efficiently as possible. This often leads to much higher coordination numbers. In NaCl, each ion has 6 bonds and all bond angles are 90°. In CsCl the coordination number is 8. By comparison carbon typically has a maximum of four bonds.
Ionic character in covalent bonds can be directly measured for atoms having quadrupolar nuclei . These nuclei are generally objects of NQR nuclear quadrupole resonance and NMR nuclear magnetic resonance studies. Interactions between the nuclear quadrupole moments Q and the electric field gradients are characterized via the nuclear quadrupole coupling constants
- QCC = e2qzzQ/h
Read Also: Chapter 10 Glencoe Geometry
What Is An Ionic Bond Or Electrovalent Bond
An ionic bond is defined as that bond between a metal and a non-metal which is responsible to hold the oppositely charged ions by the strong electrostatic force of attraction. The bond formed as a result of the transference of electrons from the outermost shell of metal to the outermost shell of a non-metal is alternatively known as an electrovalent bond.
There are two essential factors for Ionic Bond formation: 1. the metals participating in an ionic bond formation should have low Ionization Potential and 2. the non-metals participating in an ionic bond formation should have high Electron Affinity. Ionization Potential is the energy required to remove an electron from the outermost shell of an isolated gaseous atom. And finally, electron affinity is defined as the energy released in addition of an electron to the outermost shell of an isolated gaseous atom.
Formation Of Ionic Bonding
Lets look at the periodic table. Chlorine, element 17, for example, has one too few electrons. It wants to have 18, but it only has 17. Itll do almost anything it can to gain that electron. By the same token, sodium, which is element 11, has one too many electrons, and its going to do almost anything it can to lose an electron.
So, imagine what happens when a chlorine atom meets a sodium atom. Well, naturally the sodium says, Here, take my electron. I dont want it. And so, the sodiums happy it has 10 chlorines happy, it has 18. And something else happens in the process.
When sodium gives up that electron, it now has 11 positive charges in its nucleus, but only 10 electrons, so its a +1 ion. And the chlorine, it has 17 positive charges in its nucleus, but now it has 18 electrons, so its a -1 ion. You have a +1 ion, a -1 ion, they see each other and they say, Ah-ha, electrostatic attraction, and they bond. This is the formation of an ionic bond.
This is a transcript from the video series The Joy of Science. Watch it now, on Wondrium.
Don’t Miss: Value Of Kw At 25 Degrees C
Ionic Bonding In Nacl
- In NaCl, the ionic bond is formed between the metal ion, Na+, and the non-metal ion, Cl.
- The sodium atom has the configuration of 1s2 2s2 2p6 3s1, indicating that it has a single valence electron in the outermost shell.
- The atom tends to lose one electron in order to achieve a stable electronic configuration.
- The chlorine atoms being a non-metal has the configuration of 1s1 2s2 2p6, and it tends to accept one electron in order to achieve a stable electronic configuration.
- During the formation of the bond, the sodium atom loses one electron, resulting in the formation of sodium cation whereas the chlorine atom accepts one electron to form a chlorine anion.
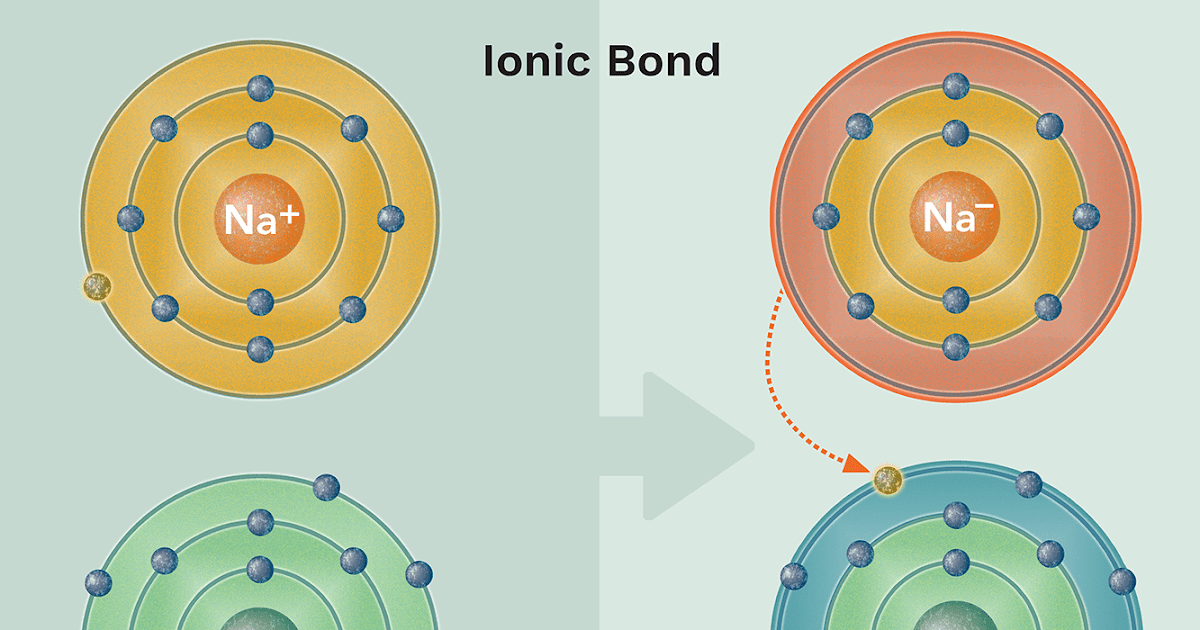
Figure: Ionic Bonding Sodium readily donates the solitary electron in its valence shell to chlorine, which needs only one electron to have a full valence shell. The opposite electrical charges of the resulting sodium cation and chloride anion result in the formation of a bond of attraction called an ionic bond. Image Credit: Openstax.