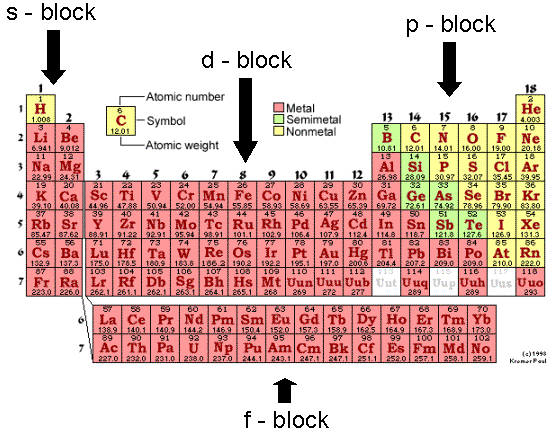
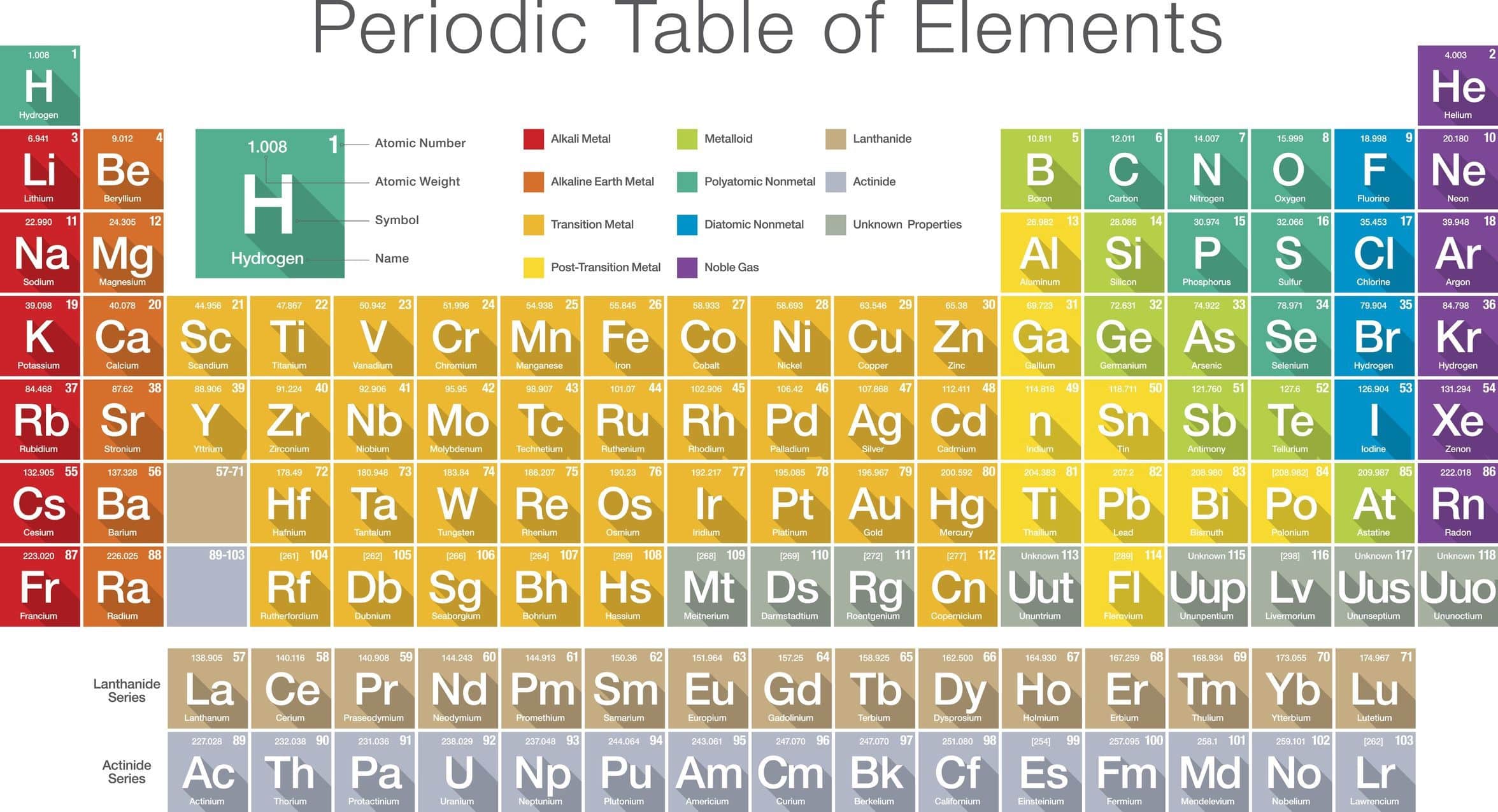
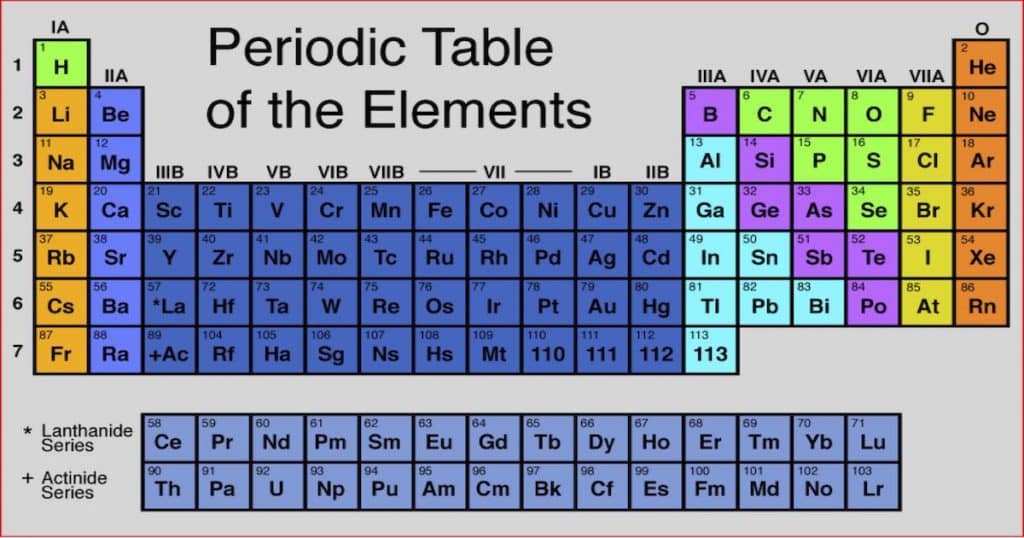
Chemistry As A Physical Science
Chemistry is typically considered a physical science, as defined by the Encyclopedia Britannica , because the study of chemistry does not involve living things. Most of the chemistry involved in research and development, such as making new products and materials for customers, falls within this purview.
But the distinction as a physical science becomes a bit blurry in the case of biochemistry, which explores the chemistry of living things, according to the Biochemical Society . The chemicals and chemical processes studied by biochemists are not technically considered “living,” but understanding them is important to understanding how life works.
Examples Of Chemistry In A Sentence
chemistrychemistrychemistrychemistry The New York Review of Bookschemistry USA TODAYchemistryVarietychemistry WSJchemistryclevelandchemistry SPINchemistryBostonGlobe.comchemistryWired
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘chemistry.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
What Chemistry Is And Why You Should Study It
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
If you look ‘chemistry’ up in Webster’s Dictionary, you’ll see the following definition:
“chem·is·try n., pl. -tries. 1. the science that systematically studies the composition, properties, and activity of organic and inorganic substances and various elementary forms of matter. 2. chemical properties, reactions, phenomena, etc.: the chemistry of carbon. 3. a. sympathetic understanding rapport. b. sexual attraction. 4. the constituent elements of something the chemistry of love. .”
A common glossary definition is short and sweet: Chemistry is the “scientific study of matter, its properties, and interactions with other matter and with energy”.
You May Like: Algebra 1 Eoc Practice Test
Amyris Publishes Second Annual Esg Report
EMERYVILLE, Calif., Aug. 31, 2022 /PRNewswire/ — Amyris, Inc. , a leading synthetic biotechnology company accelerating the world’s transition to sustainable consumption through its Lab-to-MarketTM technology platform and clean beauty consumer brands, published today its 2021 Environmental, Social, and Governance report, highlighting the company’s commitment to leading the worlds transition to clean, sustainable chemistry.
“Sustainability is at the core of everything we do, and as we grow, so too will the benefits for people and the planet,” said John Melo, CEO and President of Amyris. “The future of health, wellness and beauty is clean chemistry, and this report provides deeper transparency and accountability around our bold vision. Our mission to accelerate the world’s transition to sustainable consumption has been in place from the beginning. Our record third quarter to date consumer revenue and full year record demand for our consumer brands is a great example of the rate consumers are shifting to a more sustainable future and healthier living for them and our planet.”
Amyris’ 2021 ESG Report primarily covers the 2021 calendar year. Key highlights from the 2021 ESG Report include:
You may download a copy of the 2021 ESG Report here
About Amyris
Amyris, the Amyris logo, and Lab-to-Market, are trademarks or registered trademarks of Amyris, Inc. or its subsidiaries in the U.S. and/or other countries.
Forward-Looking Statements
Its Qualitative And Quantitative
The study of chemistry spans the range from qualitative in focus to quantitative. The more qualitative chemist might work on synthesizing a new compound used in medicine, for example, while the more quantitative work can seem much like physics applied to the microscopic level of atoms and molecules.
Recommended Reading: How To Calculate Tension In Physics
Royal Commission On Agriculture
Lavoisier urged the establishment of a Royal Commission on Agriculture. He then served as its Secretary and spent considerable sums of his own money in order to improve the agricultural yields in the , an area where farmland was of poor quality. The humidity of the region often led to a blight of the rye harvest, causing outbreaks of among the population. In 1788 Lavoisier presented a report to the Commission detailing ten years of efforts on his experimental farm to introduce new crops and types of livestock. His conclusion was that despite the possibilities of agricultural reforms, the tax system left tenant farmers with so little that it was unrealistic to expect them to change their traditional practices.
Assigning R And S Configuration: Steps And Rules
To assign the absolute configuration, we need to first locate the carbon with four different groups connected to it. These are called chirality centers .
In our molecule, we only have one carbon with four different groups and that is the one with the bromine and we are going to assign the absolute configuration of this chiral center.
For this, you need to follow the steps and rules of the Cahn-Ingold-Prelog system.
Step 1:
Give each atom connected to the chiral center a prioritybased on its atomic number. The higher the atomic number, the higher the priority.
So, based on this, bromine gets priority one, the oxygen gets priority two, the methyl carbon is the third and the hydrogen is the lowest priority-four:
Step 2:
Draw an arrow starting from priority one and going to priority two and then to priority 3:
If the arrow goes clockwise, like in this case, the absolute configuration is R.
As opposed to this, if the arrow goes counterclockwise then the absolute configuration is S.
As an example, in the following molecule, the priorities go Cl > N > C > H and the counterclockwise direction of the arrow indicates an S absolute configuration:
So, remember: Clockwise R, Counterclockwise S.
Now, lets see what would be the absolute configuration of the enantiomer:
And this is another important thing to remember:
All the chirality centers in enantiomers are inverted .
The lowest priority must point away from the viewer.
Also Check: What Does Convenience Mean In Math
Lavoisier As A Social Reformer
Research benefitting the public good
While Lavoisier is commonly known for his contributions to the sciences, he also dedicated a significant portion of his fortune and work toward benefitting the public. Lavoisier was a humanitarianhe cared deeply about the people in his country and often concerned himself with improving the livelihood of the population by agriculture, industry, and the sciences. The first instance of this occurred in 1765, when he submitted an essay on improving urban street lighting to the French Academy of Sciences.
Three years later in 1768, he focused on a new project to design an aqueduct. The goal was to bring water from the into Paris so that the citizens could have clean drinking water. But, since the construction never commenced, he instead turned his focus to purifying the water from the Seine. This was the project that interested Lavoisier in the chemistry of water and public sanitation duties.
Additionally, he was interested in air quality and spent some time studying the health risks associated with gunpowder’s effect on the air. In 1772, he performed a study on how to reconstruct the Hôtel-Dieu hospital, after it had been damaged by fire, in a way that would allow proper ventilation and clean air throughout.
Sponsorship of the sciences
Lavoisier had a vision of public education having roots in “scientific sociability” and philanthropy.
What Is The Difference Between A Shell And An Orbital
A shell in an atom is a set of subshells of the same quantum number theory, n. Orbitals contain two electrons each, and electrons are part of the same orbital in an orbital of the same definition of size, angular momentum size, and magnetic quantum number.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Read Also: Geometry Assessment Book Chapter 1 Test B
What Does It Mean If Delta H Is Positive Or Negative
As mentioned above, a negative Delta H is associated with a decrease in net energy, and a positive Delta H indicates an increase in total power.
Delta H being negative suggests that the reaction gives off heat from reactants to products, which is considered favorable. Furthermore, a negative Delta H means that the heat flows from a system to its surroundings.
When Delta H is negative, its regarded as an exothermic reaction. This is because the enthalpy of the products is lower than that of the reactants in a system.
The enthalpies in a reaction are less than zero and therefore considered exothermic. In contrast, a PositiveDelta H indicates the heat flowing from its surrounding into a system. This is an endothermic reaction where heat or energy is gained.
What Is A Chemical Equation
nomenclature
A chemical equation is an expression of a chemical process. For example:
In this equation, AgNO3 is mixed with NaCl. The equation shows that the reactants react through some process to form the products . Since they undergo a chemical process, they are changed fundamentally.
Often chemical equations are written showing the state that each substance is in. The sign means that the compound is a solid. The sign means the substance is a liquid. The sign stands for aqueous in water and means the compound is dissolved in water. Finally, the sign means that the compound is a gas.
Coefficients are used in all chemical equations to show the relative amounts of each substance present. This amount can represent either the relative number of molecules, or the relative number of moles . If no coefficient is shown, a one is assumed.
On some occasions, a variety of information will be written above or below the arrows. This information, such as a value for temperature, show what conditions need to be present for a reaction to occur. For example, in the graphic below, the notation above and below the arrows shows that we need a chemical Fe2O3, a temperature of 1000 degrees C, and a pressure of 500 atmospheres for this reaction to occur.
The graphic below works to capture most of the concepts described above:
Recommended Reading: How Long Is Ap Physics 1 Exam
Ksp Chemistry: Complete Guide To The Solubility Constant
Are you learning chemistry but dont quite understand the solubility product constant or want to learn more about it? Not sure how to calculate molar solubility from $K_s_p$? The solubility constant, or $K_s_p$, is an important part of chemistry, particularly when youre working with solubility equations or analyzing the solubility of different solutes. When you have a solid grasp of $K_s_p$, those questions become much easier to answer!
In this $K_s_p$ chemistry guide, well explain the $K_s_p$ chemistry definition, how to solve for it , which factors affect it, and why its important. At the bottom of this guide, we also have a table with the $K_s_p$ values for a long list of substances to make it easy for you to find solubility constant values.
Metalloproteins And Inorganic Cofactors
Metalloproteins in which the active site is a transition metal ion often coordinated by sulfur atoms of cysteine residues are essential components of enzymes involved in electron transfer processes. Examples include plastocyanin and nitrous oxide reductase . The function of these enzymes is dependent on the fact that the transition metal ion can undergo redox reactions. Other examples include many zinc proteins, as well as ironsulfur clusters. Most pervasive are the ferrodoxins, which serve as electron shuttles in cells. In bacteria, the important nitrogenase enzymes contains an FeMoS cluster and is a catalyst that performs the important function of nitrogen fixation, converting atmospheric nitrogen to ammonia that can be used by microorganisms and plants to make proteins, DNA, RNA, alkaloids, and the other organic nitrogen compounds necessary for life.
- Easiness of electron flow in a cluster provides catalytic effect of a respective enzyme.
Don’t Miss: What Is Visual Information Processing In Psychology
Examples For A Positive Or Negative Delta H:
An example to help better understand positive or negative Delta H conditions is: When water changes from liquid to solid, Delta H is considered harmful as the water emits heat into the surroundings.
However, when water changes from liquid to gas, Delta H is considered positive as it gains or absorbs heat from its surroundings. Moreover, 36 kJ of energy is supplied through an electric heater immersed in water. In this case, the enthalpy of the water will increase by 36 kJ, and H will be equal to +36 kJ.
This example confirms the notion that Delta H is positive when energy is gained from surroundings in the form of heat.
What Is Delta H In Chemistry
To understand Delta S better, lets look at Delta H first. It is used to describe whether a system absorbs or emits heat. In contrast to entropy, enthalpy measures the total energy within a particular system.
Therefore, if the change in enthalpy or Delta H is positive, that indicates an increase in the total amount of power within the system. On the other hand, if Delta H or enthalpy is negative, this is associated with a decrease in the total energy held within a system.
Don’t Miss: What Is Biosphere In Biology
Solving For $k: S: P$ With Solubility
In order to calculate a value for $K_s_p$, you need to have molar solubility values or be able to find them.
Question: Determine the $K_s_p$ of AgBr , given that its molar solubility is 5.71 x $10^^7$ moles per liter.
First, we need to write out the two equations.
AgBr $Ag^$ + $Br^$
$K_s_p$ =
Now, since in this problem we’re solving for an actual value of $K_s_p$, we plug in the solubility values we were given:
$K_s_p$ = = 3.26 x $10^^13$
The value of $K_s_p$ is 3.26 x $10^^13$
Relating Chemistry To Other Sciences
An important point to remember is that chemistry is a science, which means its procedures are systematic and reproducible and its hypotheses are tested using the scientific method. Chemists, scientists who study chemistry, examine the properties and composition of matter and the interactions between substances. Chemistry is closely related to physics and to biology. Chemistry and physics both are physical sciences. In fact, some texts define chemistry and physics in exactly the same way. As is true for other sciences, mathematics is an essential tool for the study of chemistry.
Don’t Miss: Predict The Geometry And Polarity Of The Cs2 Molecule
Gameplay Fixes And Adjustments
This update marks the beginning of another brand-new Season. For this update, we placed on our emphasis on improving the realism and competitiveness of user matches. As such, we have made a wide variety of enhancements and adjustments based on the themes of “playability”, “player individuality” and “proper game balance”. Let’s have a more in-depth look at some of the improvements we have made with this update.
Why Study Chemistry
Because it involves math and equations, many people shy away from chemistry or are afraid it’s too difficult to learn. However, understanding basic chemical principles is important, even if you don’t have to take a chemistry class for a grade. Chemistry is at the heart of understanding everyday materials and processes. Here are some examples of chemistry in daily life:
You May Like: Glencoe Algebra 2 Chapter 5 Answer Key
What Do Chemists Do
Chemists work in a variety of fields, including research and development, quality control, manufacturing, environmental protection, consulting and law. They can work at universities, for the government or in private industry, according to the ACS .
Here are some examples of what chemists do:
Research and development
In academia, chemists performing research aim to further knowledge about a particular topic, and may not necessarily have a specific application in mind. Their results, however, can still be applied to relevant products and applications.
In industry, chemists in research and development use scientific knowledge to develop or improve a specific product or process. For example, food chemists improve the quality, safety, storage and taste of food pharmaceutical chemists develop and analyze the quality of drugs and other medical formulations and agricultural chemists develop fertilizers, insecticides and herbicides necessary for large-scale crop production.
Sometimes, research and development may not involve bettering the product itself, but rather the manufacturing process involved in making that product. Chemical engineers and process engineers devise new ways to make the manufacturing of their products easier and more cost effective, such as increasing the speed and/or yield of a product for a given budget.
Environmental protection
Writing $k: S: P$ Expressions
Below is the solubility product equation which is followed by four $K_s_p$ chemistry problems so you can see how to write out $K_s_p$ expressions.
For the reaction $A_aB_b$ $aA^b^$ + $bB^a^$
The solubility expression is $K_s_p$= $^a$ $^b$
The first equation is known as a dissociation equation, and the second is the balanced $K_s_p$ expression.
For these equations:
- A and B represent different ions and solids. In these equations, they are also referred to as “products”.
- a and b represent coefficients used to balance the equation
- and indicate which state the product is in
- Brackets stand for molar concentration. So represents the molar concentration of AgCl.
In order to write $K_s_p$ expressions correctly, you need to have a good knowledge of chemical names, polyatomic ions, and the charges associated with each ion. Also, the key thing to be aware of with these equations is that each concentration is raised to the power of its coefficient in the balanced $K_s_p$ expression.
Lets look at a few examples.
$PbBr_2$ $Pb^2^$ + $2Br^$
$K_s_p$= $$ $^2$
In this problem, dont forget to square the Br in the $K_s_p$ equation. You do this because of the coefficient 2 in the dissociation equation.
CuS $Cu^$ + S¯
$K_s_p$=
$Ag_2CrO_4$ 2$Ag^$ + $CrO_4^2^$
$K_s_p$= $^2$
$Cu_3$ $^2$ $3Cu^2^$ + $2PO_4^3^$
$K_s_p$ = $^3$ $^2$
Also Check: What Is On The Sat Subject Test Math Level 2
Team Playstyle Long Ball
The “Long Ball” Team Playstyle is an embodiment of the renowned “Route 1 football” tactic. For this Team Playstyle, long balls are hoofed towards a solitary target at the front line in hopes of contesting and retaining possession high up the pitch for quick attacking opportunities. However, an issue arose where the midfielders would position themselves higher up the field on the receiving end of a long ball, making it difficult for the targeted player to contest and keep the ball. This also led to a higher risk of being exposed to counter attacks in cases where the long balls were intercepted. To address this issue, we have adjusted the positioning of the midfield players. This will now make it easier for the front-line target to keep the contested ball.