How Does Carbon Monoxide Affect The Environment
U.S. EPA conducted an extensive literature search as part of the review of the National Ambient Air Quality Standard for Carbon Monoxide that was completed in 2011, and did not identify any evidence for ecological effects of CO at levels at or near ambient. Consequently, there is no secondary standard for CO.
CO contributes indirectly to climate change because it participates in chemical reactions in the atmosphere that produce ozone, which is a climate change gas. CO also has a weak direct effect on climate. For these reasons, CO is classified as a short-lived climate forcing agent, prompting CO emission reductions to be considered as a possible strategy to mitigate effects of global warming.
Basic Sciences And Homeostasiscarbon Monoxide: Chemistry Role Toxicity And Treatment
Carbon Monoxide is a product of the incomplete combustion of organic materials. It is also produced endogenously in animals by haemoxygenase enzymes. Inducable haemoxygenase is involved in the breakdown of haeme to biliverdin. Constitutive haemoxygenase produces CO at a cellular level, where it acts as a neurotransmitter. It has a role in hyperpolarizing excitable membranes, and appears to be important in the formation and maintenance of memory.
CO intoxication is not uncommon, and may result in a thousand deaths per year in Britain. Most cases are due to deliberate self poisoning or to faulty domestic heating appliances. Two iatrogenic sources have been investigated. Intraperitoneal diathermy in a carbon dioxide atmosphere has not been found to cause dangerous intoxication. Excessive dehydration of carbon dioxide absorbing salts in anaesthetic circuits has been shown to be dangerous.
- Previous article in issue
Bond Polarity And Oxidation State
Theoretical and experimental studies show that, despite the greater electronegativity of oxygen, the dipole moment points from the more-negative carbon end to the more-positive oxygen end. The three bonds are in fact polar covalent bonds that are strongly polarized. The calculated polarization toward the oxygen atom is 71% for the -bond and 77% for both -bonds.
The oxidation state of carbon in carbon monoxide is +2 in each of these structures. It is calculated by counting all the bonding electrons as belonging to the more electronegative oxygen. Only the two non-bonding electrons on carbon are assigned to carbon. In this count, carbon then has only two valence electrons in the molecule compared to four in the free atom.
Also Check: What Is Fk In Physics
Using Gas Detectors To Measure Co Vs Co2
Regardless of what industry you work in, leaks and overexposure to both gases can occur around you each and every day. Recently publicized fatalities involving both CO2 and CO have refocused attention on the need to accurately and effectively detect and monitor for the presence of gases.
Understanding the gases and being able to prevent potential injuries and hazards from occurring is the best preventive first step you can take.
When it comes to choosing the right gas detector for the workplace, a single-gas CO detector will not measure CO2 levels, and vice-versa. Gas detectors are built from a specific sensing technology and principle which is specific for being able to measure each gas.
The bright side is that there are a few options when it comes to the best gas detectors for carbon monoxide or carbon dioxide. The most important factor is that you can understand the environment that you are measuring and know what gas you will need to be monitoring.
Below, we have listed our top devices for each CO2 vs. CO gas.
|
Carbon Dioxide Gas Detectors |
Carbon Monoxide Gas Detectors |
Faqs About Carbon Monoxide
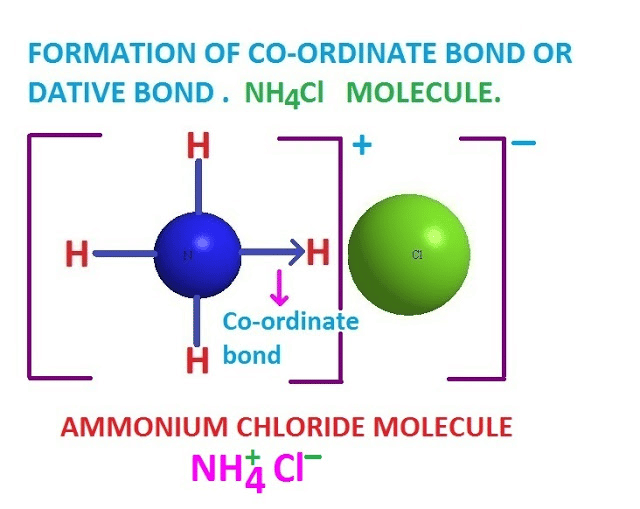
Q.1. What is the impact of carbon monoxide on the human body?
Answer. Carbon monoxide has positive and negative human effects. As we know, that increased levels of carbon monoxide in red blood cells reduce the amount of oxygen haemoglobin carries in the body. The result is that vital organs, such as the brain, the heart, and the nervous tissues, cannot function actively due to a lack of oxygen present in the body.
Q.2. Why is carbon monoxide considered harmful?
Answer. Carbon monoxide is toxic when inhaled because it displaces the oxygen present in the blood and eliminates oxygen from the heart, brain and other vital organs. Within minutes, immense amounts of CO can invade your body without warning, causing you to lose consciousness and suffocate. CO toxicity is also a known particular threat to the fetus.
Q.3. Is it possible to smell carbon monoxide?
Answer. Carbon monoxide is a colourless, odourless gas produced from a carbon-containing combustion material. You cannot smell it or taste it, but research shows that it can kill a person when they come in contact with higher concentrations. It is known to be severely toxic. CO is also known as the silent killer as it is odourless, tasteless and colourless.
Q.4. What is carbon monoxide poisoning?
You May Like: Why Teach Geography In Primary Schools
Calculating The Coordination Number
Here are the steps for identifying the coordination number of a coordination compound.
What Are The Main Uses Of Carbon Dioxide And Carbon Monoxide Detection
Carbon Dioxide sensing is crucial for a number of different industries, including HVAC, landfill, horticulture, controlled atmosphere storage and packaging, metal heat treatments, TOC and many more. Each of these applications would not be possible without precise CO2 measurement. If you are interested in finding out more about specific applications of CO2 sensing, why not have a browse of the following articles
You May Like: Pre Algebra Book Mcgraw Hill
What Does A Base Do In Chemistry
A base is very chemically reactive due to its charge imbalance. Depending on the type, a base has excess negatively charged ions when itâs dissolved in water. These excess ions tend to attract positively charged ions, particularly hydrogen ions from acids.
Many bases contain alkali and alkaline earth metals, such as sodium, potassium, magnesium and calcium, which easily form salts with halogens and other negatively charged components of acids.
Consider the reaction between copper hydroxide and sulphuric acid:
Cu2 + H2SO4 â CuSO4 + 2H2O
In this reaction, two molecules of water and one molecule of copper sulphate are produced for every molecule of copper hydroxide and sulphuric acid. Under ideal conditions when the reaction is perfectly balanced, the products will have neutral pH levels.
Aside from the defining chemical characteristics, here are some of the other general properties of bases:
- Strong bases are highly caustic and can easily dissolve organic matter, particularly fats and oils
- They react violently with acidic substances, producing high temperatures
- The positive and negative ions dissociate when dissolved in water
- Bases change the colours of indicators â for example, they can turn red litmus paper blue and change methyl orange to yellow
- Bases have a bitter taste, whereas acids have a distinctly sour taste
Co And Co2 Whats The Same
- Both are made from carbon and oxygen molecules
- Both are colorless, tasteless and odorless gases
- Both are in the air we breath
- Both are released during combustion
- Both are important industrial gases
- Both are potentially deadly and can cause severe health problems
While they both have the word “carbon” in their name, -monoxide refers to the bond between a single carbon molecule and a single oxygen molecule while -dioxide refers to the bond between a single carbon molecule and two oxygen molecules, . In other words, CO is C+O while CO2 is O+C+O.
Both carbon dioxide and carbon monoxide are colorless, odorless and tasteless gases. However, some describe the odor of high levels of CO2 as acidic or bitter.
While both CO and CO2 are potentially deadly, this happens at vastly different concentrations. While 35 ppm of CO is quickly life threatening, it takes more than 30,000 ppm of CO2 to reach the same risk level.
Compressed carbon dioxide and carbon monoxide are both important industrial gases. For example, CO2 is used to carbonate beverages and to increase plant growth in indoor greenhouses. CO is used during the manufacturing of iron and nickel as well as the production of methanol. In spite of their molecular similarity, they both behave very differently when interacting with other molecules.
Recommended Reading: Bridge To Algebra Answer Sheet
What Is Reaction Condition Of Cao Reacts With Co2
Temperature: room temperature
Explanation: The ideal environmental conditions for a reaction, such as temperature, pressure, catalysts, and solvent. Catalysts are substances that speed up the pace of a chemical reaction without being consumed or becoming part of the end product. Catalysts have no effect on equilibrium situations.
Production Of Carbon Monoxide
There are several methods for producing carbon monoxide
1)In Laboratory
The simple method for producing carbon monoxide in the laboratory is dehydrating the formic acid or oxalic acid, e.g. concentrated sulphuric acid. Another simple technique is to heat an intimate mixture of powdered zinc metal and calcium carbonate that releases CO and leaves zinc oxide and calcium oxide behind:
Silver nitrate and iodoform also produce carbon monoxide:
Metal oxalate salts also release CO when heating, leaving carbonate as a by-product:
2)Industrial Production
The primary industrial source of CO is the producer gas, a mixture mainly consisting of carbon monoxide and nitrogen, produced by burning carbon in the air at high temperatures when carbon is abundant. In the oven, the air flows into the coke bed. \ produced initially is balanced with the remaining hot carbon to emit CO. The reaction of \ with carbon to release CO is known as the Boudouard reaction. CO is the predominant element produced when heated over \:
Another method is water gas, a combination of hydrogen and carbon monoxide created by the endothermic reaction of steam and carbon:
Read Also: What Does G Mean In Physics
Carbon Monoxide And Its Various Properties
Students are taught about the hazardous effects of carbon monoxide since the fifth grade, however, the harmful effects of carbon monoxide are further discussed in depth in eighth grade in the chapter pollution of air and water. This chapter mainly deals with the harmful gases that cause air and water pollution, it talks about how gases like carbon monoxide are negatively impacting the standard of living among citizens, and how a great number of people suffer from respiratory diseases due to the excess of these gases in the environment.
There are various chemicals that contaminate the air and those are called air pollutants. The source of such chemicals is usually from natural sources like smoke and dust that arise from forest fires or volcanic eruptions. Certain man-made activities also add up to the air pollutants which are then released into the air like certain factories, power plants, automobile exhaust, and burning of firewood. These are the kinds of activities that do not carefully dispose of their waste.
Along with carbon monoxide, there are numerous gases and chemicals that pollute the air like nitrogen oxides, carbon dioxide, methane, and sulphur dioxide. These are some of the major pollutants in the air and can cause deadly diseases in living beings.
Carbon Monoxide In The Atmosphere
Carbon monoxide, though thought of as a pollutant today, has always been present in the atmosphere, chiefly as a product of volcanic activity. It occurs dissolved in molten volcanic rock at high pressures in the earth’s mantle. Carbon monoxide contents of volcanic gases vary from less than 0.01% to as much as 2% depending on the volcano. It also occurs naturally in bushfires. Because natural sources of carbon monoxide are so variable from year to year, it is extremely difficult to accurately measure natural emissions of the gas.
Carbon monoxide has an indirect radiative forcing effect by elevating concentrations of methane and troposphericozone through chemical reactions with other atmospheric constituents ” rel=”nofollow”> radical, OH.) that would otherwise destroy them. Through natural processes in the atmosphere, it is eventually oxidized to carbon dioxide. Carbon monoxide concentrations are both short-lived in the atmosphere and spatially variable.
Anthropogenic CO from automobile and industrial emissions may contribute to the greenhouse effect and global warming. In urban areas carbon monoxide, along with aldehydes, reacts photochemically to produce peroxy radicals. Peroxy radicals react with nitrogen oxide to increase the ratio of NO2 to NO, which reduces the quantity of NO that is available to react with ozone. Carbon monoxide is also a constituent of tobacco smoke.
Recommended Reading: Using Sacred Geometry To Manifest
Chemical Reactions Of Co2
CO2 is a potent electrophile having an electrophilic reactivity that is comparable to benzaldehyde or strong ,-unsaturated carbonyl compounds. However, unlike electrophiles of similar reactivity, the reactions of nucleophiles with CO2 are thermodynamically less favored and are often found to be highly reversible. Only very strong nucleophiles, like the carbanions provided by Grignard reagents and organolithium compounds react with CO2 to give carboxylates:
In metal carbon dioxide complexes, CO2 serves as a ligand, which can facilitate the conversion of CO2 to other chemicals.
The reduction of CO2 to CO is ordinarily a difficult and slow reaction:
- CO2 + 2 e + 2H+ CO + H2O
use the energy contained in sunlight to simple sugars from CO2 absorbed from the air and water:
- n CO2 + nHn + nO2
The redox potential for this reaction near pH 7 is about 0.53 V versus the standard hydrogen electrode. The nickel-containing enzyme carbon monoxide dehydrogenase catalyses this process.
Carbon Monoxide And Selenocyanate
A crystal structure of the lo-CO state was obtained by inhibiting the enzyme under turnover and subsequently isolating the protein .153 The 1.5 Å resolution structure showed the loss of 2-bridging sulfide S2B with a CO ligand in its place bridging Fe2 and Fe6. When the CO was removed under continued turnover, the sulfide returned to its original position to give the resting state of the cofactor. The location of the displaced sulfide is unclear. This was the first structural evidence that bonds to the belt sulfide groups may break. However, it should be remembered that the observed CO species are not on the pathway to N2 reduction.
Fig. 14. Crystallographically characterized structures of FeMoco with substitution at the S2B site.
Recommended Reading: Taking Algebra In 8th Grade
Advent Of Industrial Chemistry
Carbon monoxide gained recognition as an invaluable reagent in the 1900s. Three industrial processes illustrate its evolution in industry. In the FischerTropsch process, coal and related carbon-rich feedstocks are converted into liquid fuels via the intermediacy of CO. Originally developed as part of the German war effort to compensate for their lack of domestic petroleum, this technology continues today. Also in Germany, a mixture of CO and hydrogen was found to combine with olefins to give aldehydes. This process, called hydroformylation, is used to produce many large scale chemicals such as surfactants as well as specialty compounds that are popular fragrances and drugs. For example, CO is used in the production of vitamin A. In a third major process, attributed to researchers at Monsanto, CO combines with methanol to give acetic acid. Most acetic acid is produced by the Cativa process. Hydroformylation and the acetic acid syntheses are two of myriad carbonylation processes.
Supercritical Co2 As Solvent
Liquid carbon dioxide is a good solvent for many lipophilicorganic compounds and is used to remove caffeine from coffee. Carbon dioxide has attracted attention in the pharmaceutical and other chemical processing industries as a less toxic alternative to more traditional solvents such as organochlorides. It is also used by some dry cleaners for this reason. It is used in the preparation of some aerogels because of the properties of supercritical carbon dioxide.
Recommended Reading: What Is Improvisation In Chemistry
Organic And Main Group Chemistry
In the presence of strong acids and water, carbon monoxide reacts with olefins to form carboxylic acids in a process known as the Koch-Haaf reaction. In the Gattermann-Koch reaction, arenes are converted to benzaldehyde derivatives in the presence of AlCl3 and HCl. Organolithium compounds, e.g. butyl lithium react with CO, but this reaction enjoys little use.
Although CO reacts with carbocations and carbanions, it is relatively unreactive toward organic compounds without the intervention of metal catalysts.
With main group reagents, CO undergoes several noteworthy reactions. Chlorination of CO is the industrial route to the important compound phosgene. With borane CO forms an adduct, H3BCO, which is isoelectronic with the acylium cation +. CO reacts with sodium to give products resulting from C-C coupling such as Na2C2O2 , and potassium to give K2C2O2 and K2C6O6 .
Oxygen And Chalcogen Compounds
Several oxides of cobalt are known. Green cobalt oxide has rocksalt structure. It is readily oxidized with water and oxygen to brown cobalt hydroxide 3). At temperatures of 600700 °C, CoO oxidizes to the blue cobalt oxide , which has a spinel structure. Black cobalt oxide is also known. Cobalt oxides are antiferromagnetic at low temperature: CoO and Co3O4 , which is analogous to magnetite , with a mixture of +2 and +3 oxidation states.
Read Also: What Does Plane Mean In Math Terms