Structure Of Cellulose N
Structure of Cellulose n
In high temperature, It can be broken down into glucose by treating with concentrated minerals acids. It is more crystalline when compared to starch. But starch goes from crystalline to amorphous transition in 60-70 degrees but cellulose, on the other hand, requires 320 degrees and a pressure of 25 Megapascal.
Can You Guess How Many People Are In This Stadium
If you attend a college or professional football game, you need a ticket to get in. It is very likely that your ticket may specify a gate number, a section number, a row, and a seat number. No other ticket can have the same four parts to it. It may have the same gate, section, and seat number, but it would have to be in a different row. Each seat is unique and allows only one occupant to fill it.
We use a series of specific numbers, called quantum numbers, to describe the location of an electron in an associated atom. Quantum numbers specify the properties of the atomic orbitals and the electrons in those orbitals. An electron in an atom or ion has four quantum numbers to describe its state. Think of them as important variables in an equation which describes the three-dimensional position of electrons in a given atom.
How Normality Can Change
Because normality references concentration with respect to the reactive species, it’s an ambiguous unit of concentration . An example of how this can work may be seen with iron thiosulfate, Fe23. The normality depends on which part of the redox reaction you’re examining. If the reactive species is Fe, then a 1.0 M solution would be 2.0 N . However, if the reactive species is S2O3, then a 1.0 M solution would be 3.0 N .
Recommended Reading: Scientific Definition Of Abiotic
When To Use Normality
There are specific circumstances when it’s preferable to use normality rather than molarity or other unit of concentration of a chemical solution.
- Normality is used in acid-base chemistry to describe the concentration of hydronium and hydroxide . In this situation, 1/feq is an integer.
- The equivalence factor or normality is used in precipitation reactions to indicate the number of ions that will precipitate. Here, 1/feq is once again and integer value.
- In redox reactions, the equivalence factor indicates how many electrons can be donated or accepted by an oxidizing or reducing agent. For redox reactions, 1/feq may be a fraction.
Normality Calculator For Concentrated Liquid Chemical

This is very handy tool for science student to make reagents for analysis. You can easily calculate normality of any concentrated acid or base liquid solution by using this normality calculator.
In conclusion, Normality or equivalent concentration of the solution indicates how many equivalent are present per liter of solution.
You May Like: Why Are There Different Branches Of Chemistry
What Does N Mean In Chemical Names
4.8/5namenomenclaturenomenclaturen
Similarly, you may ask, what is N in chemistry?
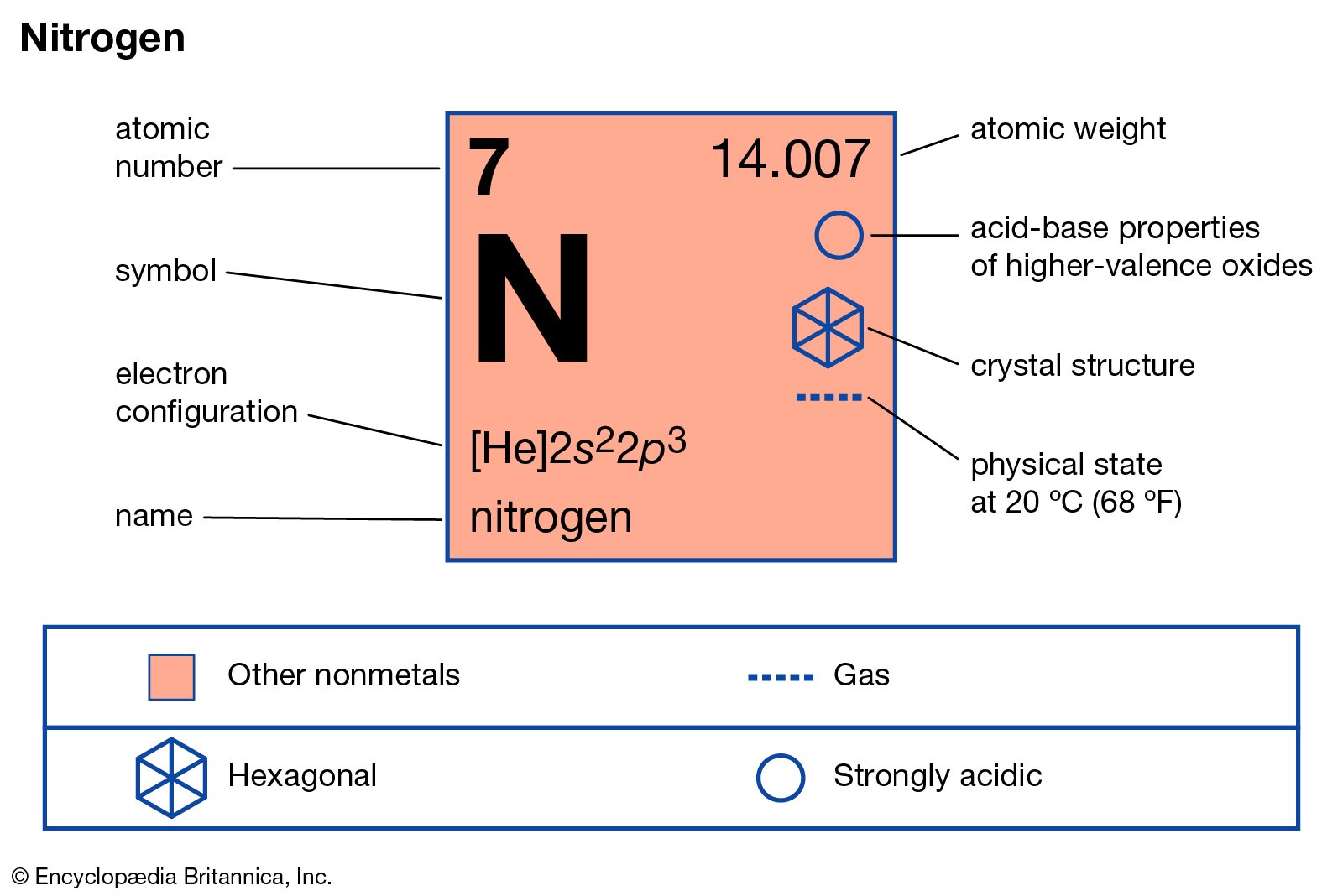
N is used for nitrogen atom. It’s atomic number is 7 and mass number is 14. It contains 7 protons and 7 electrons. The content of nitrogen gas on earth is approx. 78%.
Subsequently, question is, what is the meaning of N in N hexane? The letter n is used in front of hexane in order to differentiate the normal straight-chain hexane from its isomers.
Likewise, people ask, what does N mean when naming amines?
3. The N– prefix. When it’s used: for amines and amides. What it means: The N signifies that the substitutent is connected to the nitrogen. Example: N-methyl butylamine, N,N-dimethylformamide.
What does mean in organic chemistry?
The minus sign in parentheses here indicates the optical rotation, means the molecule rotates linear polarized light clockwise, means it rotates counterclockwise. Typically you would also write exactly which stereoisomer is meant , which is generally the more useful information.
Differences Between Normality And Molarity
Here are some key differences between normality and molarity.
| Normality | |
| It is defined as the number of gram equivalent per litre of solution. | It is defined as the number of moles per litre of solution. |
| It is used in measuring the gram equivalent in relation to the total volume of the solution. | It is used in measuring the ratio between the number of moles in the total volume of the solution. |
| The units of normality are N or eq L-1 | The unit of molarity is M or Moles L-1 |
Don’t Miss: Which Pioneer In Psychology Helped Develop The School Of Thought Called Structuralism
The Principal Quantum Number
The principal quantum number, \, designates the principal electron shell. Because n describes the most probable distance of the electrons from the nucleus, the larger the number n is, the farther the electron is from the nucleus, the larger the size of the orbital, and the larger the atom is. n can be any positive integer starting at 1, as \ designates the first principal shell . The first principal shell is also called the ground state, or lowest energy state. This explains why \ can not be 0 or any negative integer, because there exists no atoms with zero or a negative amount of energy levels/principal shells. When an electron is in an excited state or it gains energy, it may jump to the second principle shell, where \. This is called absorption because the electron is “absorbing” photons, or energy. Known as emission, electrons can also “emit” energy as they jump to lower principle shells, where n decreases by whole numbers. As the energy of the electron increases, so does the principal quantum number, e.g., n = 3 indicates the third principal shell, n = 4 indicates the fourth principal shell, and so on.
If n = 7, what is the principal electron shell?
Example \
If an electron jumped from energy level n = 5 to energy level n = 3, did absorption or emission of a photon occur?
- Answer
-
Emission, because energy is lost by release of a photon.
How To Calculate Normality From Molarity
The mole equivalents of an acid or base are calculated by determining the number of H+ or OH- ions per molecule: N = n × M
For an acid solution, n is the number of H+ ions provided by a formula unit of acid.Example: A 3 M H2SO4 solution is the same as a 6 N H2SO4 solution.
For a basic solution, n is the number of OH- ions provided by a formula unit of base.Example: A 1 M Ca2 solution is the same as a 2 N Ca2 solution.
Note:The normality of a solution is NEVER less than its molarity!
Read Also: Geometry Segment And Angle Addition Worksheet Answers
What Is Valency In Chemistry
The valency or valence of an atom or molecule described as how may hydrogen atoms it can bond with. In our example,
Hydrochloric Acid HCl have a one hydrogen atom so, the equivalents of Hydrochloric Acid is 36.46/1 = 36.46
Sulphuric Acid H2SO4 have two hydrogen atoms so, the equivalents of Sulphuric Acid is 98/2 = 49
Oxoacids Oxoanions And Oxoacid Salts
Many nitrogen oxoacids are known, though most of them are unstable as pure compounds and are known only as aqueous solution or as salts. Hyponitrous acid is a weak diprotic acid with the structure HON=NOH . Acidic solutions are quite stable but above pH 4 base-catalysed decomposition occurs via to nitrous oxide and the hydroxide anion. Hyponitrites are stable to reducing agents and more commonly act as reducing agents themselves. They are an intermediate step in the oxidation of ammonia to nitrite, which occurs in the nitrogen cycle. Hyponitrite can act as a bridging or chelating bidentate ligand.
- ArNH2 + HNO2 Cl + 2 H2O
Nitrite is also a common ligand that can coordinate in five ways. The most common are nitro and nitrito . Nitro-nitrito isomerism is common, where the nitrito form is usually less stable.
- 2 HNO3 H3 + NO3 H2O + + +
Two hydrates, HNO3·H2O and HNO3·3H2O, are known that can be crystallised. It is a strong acid and concentrated solutions are strong oxidising agents, though gold, platinum, rhodium, and iridium are immune to attack. A 3:1 mixture of concentrated hydrochloric acid and nitric acid, called aqua regia, is still stronger and successfully dissolves gold and platinum, because free chlorine and nitrosyl chloride are formed and chloride anions can form strong complexes. In concentrated sulfuric acid, nitric acid is protonated to form nitronium, which can act as an electrophile for aromatic nitration:
- HNO3 + 2 H2SO4 NO+4
- NaNO 4 +Na2O-> Na3NO4}}}
You May Like: Exponent Rules Worksheet 8th Grade
The Five Main Branches Of Chemistry
Traditionally, chemistry is broken into five main branches, according to the online chemistry textbook published by LibreText. There are also more specialized fields, such as food chemistry, environmental chemistry and nuclear chemistry, but this section focuses on chemistry’s five major subdisciplines.
Analytical chemistry involves the analysis of chemicals, and includes qualitative methods like looking at color changes, as well as quantitative methods like examining the exact wavelength of light that a chemical absorbed to result in that color change.
These methods enable scientists to characterize many different properties of chemicals, and can benefit society in a number of ways. For example, analytical chemistry helps food companies make tastier frozen dinners by detecting how chemicals in food change when they are frozen over time. Analytical chemistry is also used to monitor the health of the environment by measuring chemicals in water or soil, for example.
Biochemistry, as mentioned above, uses chemistry techniques to understand how biological systems work at a chemical level. Thanks to biochemistry, researchers have been able to map out the human genome, understand what different proteins do in the body and develop cures for many diseases.
Related: Unraveling the human genome: 6 molecular milestones
Inorganic chemistry is used to create a variety of products, including paints, fertilizers and sunscreens.
How Do We Calculate N Factor And Why Is It Useful In Calulations
I don’t understand the concept of N factor at all. Why is it important?
I know that it is something dependent upon the oxidation number, for example N factor of Na would be 1 since it has oxidation number of +1.
For acid and bases we look at the replaceable $\ce$ or $\ce$ present in them.
Apart from this, I didn’t really understand how I can use N factor for calculation or how to find the N factor for whole chemical reactions. Please correct me where I’m wrong.
There are some different applications.
For one, it tells you how many times an acid can lose a proton. For instance, you can have a diprotic molecule, with $n=2$. For instance, $\ce$, deprotonates to $\ce$, which can then go to $\ce}$.
Why is that important? Take an acidic reaction with $\ce$. If the extent of reaction is strongly forward, you would treat $\ce$ as if it had twice its concentration to take into account that it has two protons it can lose. If you only accounted for the first proton you would be underestimating this effect.
It’s also known as equivalent concentration, which might make it sound less foreign to you.
Also Check: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Main Difference Hexane Vs N
Hexane and n-hexane are organic compounds which are included in the alkane category. They are saturated hydrocarbons and contain only single bonds between all atoms. Hexane and n-hexane are also aliphatic compounds. This means these are not ring structures. These compounds are important constituents of gasoline. Therefore, the main source of hexane compounds is gasoline. Since these are comparatively small hydrocarbons, they are easily evaporated. n-hexane is the linear form of hexane. Hexane is a mixture of branched and unbranched molecules having the chemical formula C6H14. The main difference between hexane and n-hexane is that hexane has 5 structural isomers that are either branched or unbranched whereas n-hexane is an unbranched structure.
Commercial Production And Uses
Commercial production of nitrogen is largely by fractional distillation of liquefied air. The boiling temperature of nitrogen is 195.8 °C , about 13 °C below that of oxygen, which is therefore left behind. Nitrogen can also be produced on a large scale by burning carbon or hydrocarbons in air and separating the resulting carbon dioxide and water from the residual nitrogen. On a small scale, pure nitrogen is made by heating bariumazide, Ba2. Various laboratory reactions that yield nitrogen include heating ammonium nitrite solutions, oxidation of ammonia by bromine water, and oxidation of ammonia by hot cupric oxide.
Elemental nitrogen can be used as an inert atmosphere for reactions requiring the exclusion of oxygen and moisture. In the liquid state, nitrogen has valuable cryogenic applications except for the gases hydrogen, methane, carbon monoxide, fluorine, and oxygen, practically all chemical substances have negligible vapour pressures at the boiling point of nitrogen and exist, therefore, as crystalline solids at that temperature.
Read Also: What Does The P Stand For In Pemdas
Calculating Volume Of Solution
Calculate the volume of a 0.80 mol L-1 potassium bromide solution containing 1.60 moles of potassium bromide.
V = volume of solution in litres = ? L
Extract the data from the question: n = moles of solute = 1.60 mol c = molarity of solution = 0.80 mol L-1
Write the equation:
V = 1.60 ÷ 0.80 = 2.00 L
Can you apply this?
How Can You Use This
Lets summarize what weve discussed so far:
Up to Z = 20 , the rule correctly predicts:
- Orbital energy levels
The order of occupancy of the orbitals
- The physical meaning of the rule is related to the size and shape of a given orbital.
For Z > 20 :
- The rule is not able to correctly predict orbital energy levels.
- Even when we know the orbital energies, this knowledge is not sufficient to predict the order of filling. Other factors, such as d vs. s electron repulsions must be considered. .
- Although its physical meaning is no long sufficient, the rule still correctly predicts the order of filling. Except where it doesnt and we invoke exceptions.
The first point to be taken from this is that the rule is a model and that it works until it doesnt. If you choose to teach it as a model and connect it to some of the physical meaning discussed above, its a great example of how models can be both useful and also fail.
The story outlined above has the potential to be much more fulfilling for your students than Memorize the diagram, learn to use it, and youre guaranteed to get the right answer. But its a tough story to tell by just waving your hands. You need a model to tell it, and the model needs the following features:
References:
Read Also: Parallax Error Camera
Chemistry Definitions Starting With The Letter N
This chemistry dictionary offers the chemistry definitions starting with the letter N. These glossary terms are commonly used in chemistry and chemical engineering. Click the letter below to find the terms and definitions beginning with that letter.
NA number An NA number or DOT number is a North America number assigned by the United States Department of Transportation to identify a hazardous or flammable chemical. It is analogous to a UN Number, except some chemicals have an NA number, yet dont have a UN number. These additional NA numbers have the range NA8000 NA9999.
nano Nano is the prefix associated with x10-9 and is denoted by the symbol n.Example: Visible light has a range of wavelengths from 400 to 700 nanometers.
nanometer A nanometer is a unit of length equal to 1/1,000,000,000th of a meter. The symbol for millimeter is nm.1 mm = 10-9 m.
nanotechnology Nanotechnology is the study and development of materials and objects on the nanometer level of measurement. Nanotechnology typically involves materials on the atomic or molecular level. Quantum mechanical effects play a large role in the study of nanotechnology.
naphthenes Naphthenes are a class of cyclic aliphatic hydrocarbons obtained from petroleum. Naphthenes have the general formula CnH2n.Alternate Spellings: naphtheneCommon Misspellings: napthene, napthenesExample: Cyclohexane is the simplest naphthene molecule
normal There are two meanings for normal in chemistry.
N+l Rule For Atomic Electron Configurations
Requires a Wolfram Notebook System
Interact on desktop, mobile and cloud with the free Wolfram Player or other Wolfram Language products.
Do not show again
Orbitals in atomic ground-state electron configurations are filled in the order of increasing . For equal values, the orbital with the lower is most often filled first. Here is the principal quantum number and is the angular momentum quantum number , designated by the code for , respectively. The “rule,” also known as the Madelung rule or the diagonal rule, holds with only a small number of irregularities. The designation “diagonal rule” refers to the pattern of atomic orbitals shown in the graphic. The optional configuration diagram also shows the spins of the electrons in occupied orbitals. In accord with the Pauli exclusion principle, each orbital has a maximum capacity of two electrons, with opposite spins. The actual electron configurations are deduced from spectroscopic and chemical characteristics. This Demonstration covers the naturally occurring elements with atomic numbers from 1 to 92.
Also Check: Theory Of Everything Geometry Dash Song