Amorphous Sic Thin Films
The crystallization temperature of SiC thin films sputtered from SiC target is about 500°C, below which the films are amorphous. However, the stable amorphous phase is obtained in SiC thin films reactively sputtered from a Si target in a C2H2 or CH4 gas.195 Typical sputtering conditions for the deposition of amorphous SiC films are shown in Table 6.52. Although the substrate temperature is higher than the crystallization temperature of conventional RF sputtered SiC thin films, the reactively sputtered SiC thin films show the amorphous phase. Their infrared transmission spectra are shown in Fig. 6.159. The large absorption band at 800 cm1 corresponds to the fundamental lattice vibration of SiC.173 The small absorptions due to SiH and CH are superposed on the spectra. The inclusion of hydrogen atoms in sputtered SiC films stabilize the amorphous phase. The sputtering process is easy way for a fabrication of amorphous phase. Similar to the SiC, thin films of amorphous Si are also deposited by the sputtering.196 Since the amorphous thin films often include microcrystallites of nanoscale, the amorphous thin films are one of the promising materials for future application.
Table 6.52. Sputtering Conditions for the Preparation of Amorphous SiC Thin Films
| Sputtering system |
I. Szafraniak-Wiza, … D. Hesse, in, 2008
How To Perform A Simple Recrystallization Procedure
Below is a stepwise guide for completing a basic recrystallization procedure.
1. First, weigh the impure solvent and record that value. Then add the impure compound to a solvent system.
2. Heat this solvent system to your target temperature, or its boiling point. Be sure to raise the temperature in gradual increments to ensure that the rest of the process proceeds smoothly.
3. Let the solution stand without disturbance. Allow the temperature to slowly drop, reaching room temperature. If you do not maintain a gradual temperature decrease, a precipitate may form instead of the desired crystals. Additionally, do not place the flask on a hard surface, which will cause shock-cooling of your solution. Instead, either place the flask in an insulated jar or clamp it to a steady, secure device. Recrystallization is a gradual process do not become impatient as you wait and employ appropriate safety precautions.
4. To further lower the temperature, place the solution in an ice-water bath. This facilitates the formation of crystals by speeding the recrystallization reaction. Shortly after completing this step, you should observe many crystals.
5. Filter the crystals via vacuum filtration.
6. Air dry the crystals. Then weigh this recovered compound.
S To Induce Crystallization
Crystallization occurs when the solubility of a solute in solution is reduced by some means. Common methods to reduce solubility include:
a. Cooling
c. Evaporation of Solvent
d. Precipitation Through Chemical Reaction
The choice of crystallization method depends on the equipment available for crystallization, the objectives of the crystallization process and the solubility and stability of the solute in the chosen solvent.
You May Like: Ccl4 Lewis Structure Molecular Geometry
Solubility Curves Supersaturation And The Metastable Zone Width
Traditionally, crystal formation has been achieved by reducing the solubility of the solute in a saturated solution in a variety of ways.
Solubility curves are a common tool used by scientists to understand/demonstrate the relationship between solubility, temperature, and solvent type. By plotting this information, scientists can find the optimum solvent/anti-solvent, temperature, and theoretical yield for their crystallization process.
Figure 2 shows that the given material is highly soluble in Solvent A, meaning more material can be crystallized from a given volume of solvent. Conversely, the given material has a low solubility in Solvent C across all temperatures, potentially making it a good anti-solvent for this material
Statistical Analysis Of Databases And Side Chain Entropy Reduction
The structural models in the PDB have also been surveyed to identify macromolecular features that affect crystallization and thus more specifically guide mutagenesis strategies. The most developed of these approaches is the side chain entropy reduction method. The method is motivated by the observation that immobilizing side chains at crystal contacts between macromolecules has an entropic cost than can disfavor their formation. This idea is also statistically supported by an analysis of the correlation between protein structures and crystallizability . SER thus advocates mutating residues with high side chain entropies , such as lysine, to lower SCE residues, such as alanine. The approach was first successfully applied to Rho GDP-dissociation inhibitor, chosen for its high lysine and glutamate content and low crystallization propensity .
It should also be noted that while changing biochemical conditions for crystallization is straightforward, altering the macromolecule itself as SER suggests requires that it can be expressed, and therefore needs additional effort. Although it may have a dramatic impact on outcome, it is certainly not the easiest experimental route to follow.
You May Like: Does Mj Have Any Biological Kids
Topic : Water Of Crystallization
9.1.What is water of crystallization?Are crystals of salt really dry?
9.2. What happens when a salt loses its water of crystallization?
- It loses its texture, color and geometric shape.
- N.B. Salt that loses water of crystallization is called anhydrous salt.
9.3. What are efflorescent substances?
- The hydrated Salts which lose their water of crystallization when exposed to air are known as Efflorescent substances
- The process by which water is lost is called Efflorescence.
- Example : Copper Sulphate Pentahydrate loses its water molecules on exposure to air and becomes white in color.
9.4. What are Deliquescent Substances?
- Some salts absorbs moisture from atmosphere and are converted to liquid substance when exposed to air.
- These salts are Deliquescent substances and the process is called Deliquescence.
- Ex: Calcium Chloride and Magnesium Chloride becomes watery on exposure to air.
Crystallization Of Food Components
Crystallization of amorphous sugars is known to result in serious quality losses in food powders. For example, crystallization of amorphous lactose in dehydrated milk products has been observed to result in acceleration of the nonenzymatic browning reaction as well as other deteriorative changes and caking. Crystallization of lactose coincides with an increase in free fat, which presumably facilitates lipid oxidation in milk powders. Crystallization in low-moisture carbohydrate matrices, which contain encapsulated volatiles or lipids, results in a complete loss of flavor and release of lipids from the matrix. Water plasticization and depression of Tg to below the ambient temperature are responsible for crystallization of amorphous sugars in foods as a result of increased free volume and molecular mobility, decreased viscosity, and enhanced diffusion. Crystallization seems to initiate at Tg or corresponding aw and proceed with a rate determined by the temperature difference T Tg to a maximum extent also defined by the T Tg. The kinetics of crystallization of sugars and other amorphous food components at a constant temperature above Tg can be related to water content and aw, which define the rate controlling T Tg.
Gustavo Fenalti, … Vadim Cherezov, in, 2015
Recommended Reading: Did Michael Jackson Have Biological Kids
Process Simulations And Simple Optimization
Simulation results showing the effect of slurry concentration and crystallization temperature on median crystal size is given in Figure 3. Based on these results, the largest crystal size could be obtained under higher temperature and lower slurry concentration . However, this condition will result in low crystal yield and therefore knowledge of optimum operating conditions is necessary.
Figure 3. Process simulation results.
The relationships between temperature, median crystal size, and crystal yield are shown in Figure 4. It is obvious from this result that a higher temperature could lead to a bigger crystal size, but at the same time lower yield. Since a median crystal size of around 1100 m is desired, crystal yield of around 30% can be obtained by setting the crystallizer temperature to around 19.5°C.
Figure 4. Simple optimization of operating conditions.
Figure 5. gCRYSTAL flowsheet of 2-stage crystallization process.
Figure 6. Median size in the first and second crystallizer as a function of recycle rate.
J.L. Blin, … Bao-Lian Su, in, 2000
What Water Of Crystallization Means In Chemistry
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
Water of crystallization is defined as water that is stoichiometrically bound into a crystal. Crystal salts containing water of crystallization are called hydrates. Water of crystallization is also known as water of hydration or crystallization water.
Don’t Miss: What Does Amu Mean In Chemistry
Calculating Theoretical Crystallization Yield
The theoretical yield of crystallization can be calculated at various temperatures:
If a saturated solution containing 45 g of product per 100 g solvent A is cooled from 50 °C to 20 °C, then 15 g of product per 100 g of solvent will remain in solution. Therefore, 30 g of product should crystallize, allowing scientists to measure the yield/efficiency of their crystallization.
In reality, when a saturated solution is cooled, there is more solute in the solution than predicted by the solubility curve, and this is referred to as supersaturation.
As cooling continues, at a certain temperature, crystal nucleation will begin. This temperature is called the metastable limit, and the difference between this temperature and the solubility curve is known as the metastable zone width .
As can be seen from the above schematic, at low levels of supersaturation, crystals grow more quickly than they nucleate resulting in large crystal size distribution. At high supersaturation levels, nucleation dominates crystal growth, providing smaller crystals. This makes understanding and controlling supersaturation vitally important when creating crystals of a desired size and distribution.
Water Of Crystallization Nomenclature
The two methods to denote water of crystallization in molecular formulas are:
- “hydrated compound·nH2O” – For example, CaCl2·2H2O
- “hydrated compoundn” – For example, ZnCl24
Sometimes the two forms are combined. For example, SO4·H2O may be used to describe the water of crystallization of copper sulfate.
Recommended Reading: Asvab Arithmetic Reasoning Worksheets
How To Design A Crystallization Process
The design of a crystallization process that will deliver pure crystals with an optimized yield and size, involves considering a number of important elements:
- Choose an appropriate solvent
- Screen for stability and unwanted polymorphs
- Determine growth and nucleation kinetics
- Define a seeding strategy
- Optimize cooling and anti-solvent profiles
- Understand the impact of mixing and scale
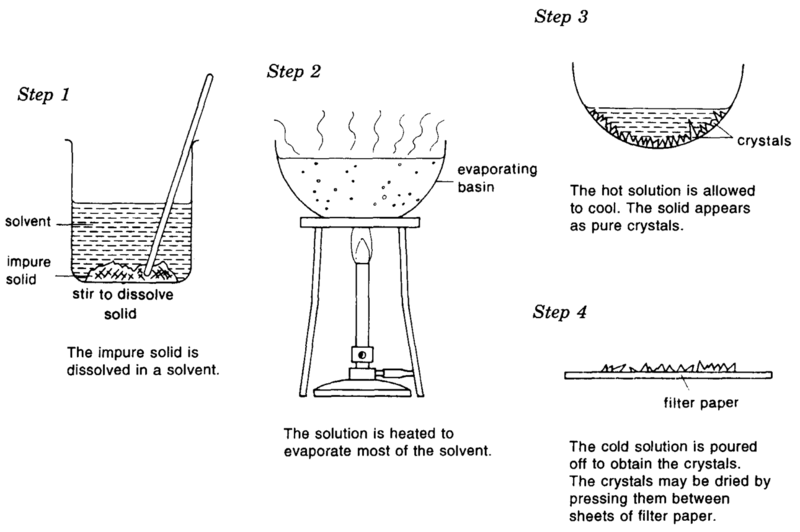
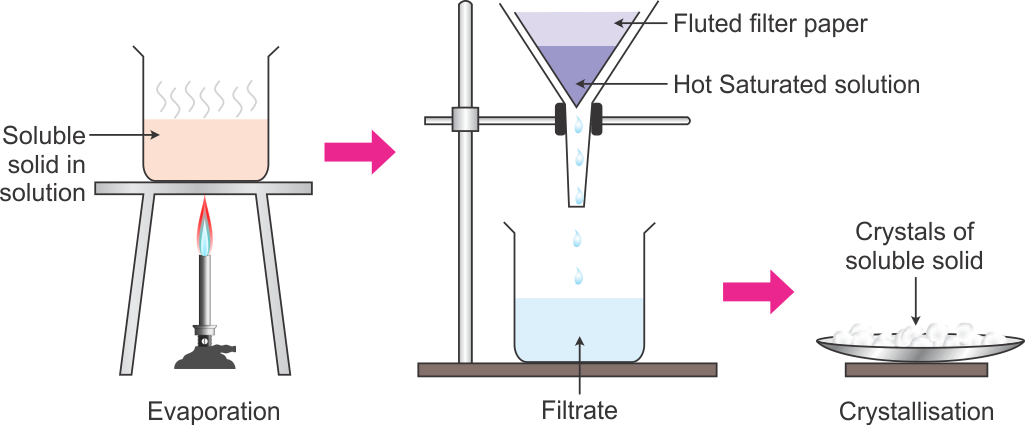
What Happens During A Crystallization
To crystallize an impure, solid compound, add just enough hot solvent to it to completely dissolve it. The flask then contains a hot solution, in which solute molecules – both the desired compound and impurities – move freely among the hot solvent molecules. As the solution cools, the solvent can no longer hold all of the solute molecules, and they begin to leave the solution and form solid crystals. During this cooling, each solute molecule in turn approaches a growing crystal and rests on the crystal surface. If the geometry of the molecule fits that of the crystal, it will be more likely to remain on the crystal than it is to go back into the solution. Therefore, each growing crystal consists of only one type of molecule, the solute. After the solution has come to room temperature, it is carefully set in an ice bath to complete the crystallization process. The chilled solution is then filtered to isolate the pure crystals and the crystals are rinsed with chilled solvent.
Read Also: Which Founding Contributors To Psychology Helped Develop Behaviorism
Analysis Of Successful Crystallization Conditions
The most accessible and immediate source of information about a macromolecule is whether a model of its structure has been obtained from crystal or not up to this point in time. Because of the intensity of the decades-long efforts at obtaining such models, that information at least reflects in part the experimental difficulty or facility of crystallizing a target.
Equipment Used For Industrial Production
Several types of equipment are used for the production of crystals on an industrial scale. Some examples follow.
1. Tank crystallizer: A hot, saturated solution is placed in an open tank and allowed to cool. Once an adequate level of crystallization is reached, the mother liquor is drained away and the crystals are removed.
2. Scraped surface crystallizer: The solution is placed in an open trough and allowed to cool with the help of a cooling jacket outside the trough. As crystals form on the inner walls of the trough, they are removed by the blades of a slow-speed agitator.
3. Forced circulating liquid evaporator-crystallizer: In this case, the solution is circulated through a heater, and then passed into the vapor space of a chamber where some of the solvent evaporates, leading to supersaturation of the remaining solution. Crystals are formed in another part of the equipment, through secondary nucleation.
Don’t Miss: How To Pass The Ap Human Geography Exam
Applications Of Recrystallization To Todays World
Recrystallization has applications that extend into the industrial, medical, and pharmaceutical industries. Techniques such as texture control, drug development, and treatment purification all involve the procedure.
But the pharmaceutical industry actually makes the most use of recrystallization procedures. Purification and separation processes are key to the isolation of different active ingredients. These steps, in turn, inform the synthesis of many different drugs and medications.
Nucleation And Growth Rates Affected By Chain Conformation
When the crystallization temperature is near the equilibrium melting temperature, n-alkanes and low molecular weight polys crystallize into once-folded or extended chain crystals. The non-integral-folded chain conformations quickly adjust to become integral-folded chain conformations at or very near the surface of the growth front. Between these two integral-folded chain crystals, the once-folded chain crystals are metastable with respect to the extended chain crystals. As such, the melting temperature of the once-folded chain crystals is lower than that of the extended chain crystals. The relationship between these growth rates is very interesting because of the concept of competing growth rates between metastable and stable phases introduced in Fig. 3.8 of Section 3 of Chapter 3.
Figure 5.15. Lineargrowth rates of the once-folded and extended chain crystals of a poly fraction with a number average molecular weight of 6 kg/mol. The melting temperature of the once-folded chain crystals is 60.3 °C and for the extended chain crystals is 63.7 °C. The crossover temperature at which both of the growth rates are identical is at 59.4 °C
Figure 5.16. Lineargrowth rates of the once-folded and extended chaincrystals of a poly fraction having methoxy end groups with a number average molecular weight of 3 kg/mol and a polydispersity of 1.02
E.B. Bond, J.E. Spruiell, in, 1998
You May Like: Algebra 1 Eoc Practice Test 2015
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
How Water Of Crystallization Forms
Many compounds are purified by crystallization from an aqueous solution. The crystal excludes many contaminants, however, water can fit within the crystalline lattice without being chemically bonded to the cation of the compound. Applying heat can drive off this water, but the process typically damages the crystalline structure. This is fine, if the goal is to obtain a pure compound. It may be undesirable when growing crystals for crystallography or other purposes.
You May Like: Who Is The Biological Father Of Elton John’s Sons
What Are The Uses Of Crystallization
Crystallization is primarily employed as a separation technique in order to obtain pure crystals of a substance from an impure mixture. Another important application of crystallization is its use to obtain pure salt from seawater. Crystallization can also be used to obtain pure alum crystals from an impure alum. In such scenarios, crystallization is known to be more effective than evaporation since it also removes the soluble impurities.
What Is The Definition Of The Term Crystallization
Crystallization can be defined as the solidification of a liquid substance into a highly structured solid whose atoms or molecules are placed in a well-defined three-dimensional crystal lattice. The smallest individual part of a crystal is called a unit cell. The crystal is made up of millions of such unit cells.
Don’t Miss: Geometry Dash Hack Iphone
How To Treat Your Crystals Once You Have Them
First and foremost: NEVER remove the solvent! Frequently, solvent molecules cocrystallize with your compound, which makes them integral parts of the crystal lattice. Removing the mother liquor from the crystals exposes the crystals to air and the volatile solvent molecules slowly evaporate from the crystal lattice, leaving empty holes. Very small holes reduce the maximum resolution the crystal diffracts to, larger holes destroy the crystal.
It is always a good idea to not change the environmental conditions for your crystals too often. When possible, leave them alone.
Laboratory Uses Of Crystallization
Crystallization is a common and useful laboratory technique. It can be used to purify substances, and can be combined with advanced imaging techniques to understand the nature of the substances crystallized. In laboratory crystallization, a substance can be dissolved into an appropriate solvent. Heat and changes in acidity can help the material dissolve. When these conditions are reversed, the materials within the solution precipitate out at different rates. If the conditions are controlled properly, pure crystals of a desired substance can be obtained.
An advanced imaging technique, called crystallography, x-rays or other high-energy beams and particles can be shot through the crystal structure of a pure substance. While this doesnt create a visible image, the rays and particles are diffracted in specific patterns. These patterns can be detected by special developing paper or electronic detectors. The pattern can then be analyzed by mathematics and computers, and a model of the crystal can be formed. The diffraction patterns are created when particles or beams are redirected by dense electron-clouds within the crystal structure. These dense areas represent the atoms and bonds present in the crystal, formed during crystallization. Using this method, scientists can recognize almost any substance based on its crystal form.
Recommended Reading: Segment Addition Postulate Find The Length Indicated