Macromolecule: Definition Types Examples
Macromolecules can be defined as large molecules composed of thousands of covalently connected atoms. These particles are very fascinating to concentrate about as they are available in nearly everything from plastics to materials and in human beings. More than 10,000 atoms make one Macromolecule. They are generally the result of more modest particles, similar to proteins, lipids, and carbs.
The structures blocks which make up macromolecules are known as monomers. Macromolecules can also refer as polymers. When word macromolecules are breaked in macro means big or large and molecule refers to its components or atoms means large atoms.
|
Table of Content |
- Industrial macromolecules
Macromolecular Structure Determines Function And Regulation
Students should be able to explain and apply core concepts of macromolecular structure and function, including the nature of biological macromolecules, their interaction with water, the relationship between structure and function, and frequently encountered mechanisms for regulating their function.The learning goals below are categorized as introductory A, intermediate B and upper C.
Biopolymers Consisting Of Regularly Repeating Units Tend To Form Helices
Just what is a helix? A helical structure consists of repeating units that lie on the wall of a cylinder such that the structure is superimposable upon itself if moved along the cylinder axis.
A helix looks like a spiral or a screw.A zig-zag is a degenerate helix.
Helices can be right-handed or left handed. The difference between the two is that:
Right-handed helices or screws advance if turned clockwise.
- Examples: standard screw, bolt, jar lid.
- Example: some automobile lug nuts.
Helical organization is an example of secondary structure.These helical conformations of macromolecules persist in solution only if they are stabilized. What might carry out this stabilization?
Recommended Reading: Geometry Mcgraw Hill Workbook Answers
Tertiary Structure In Proteins
Hydrophobic R-groups, as in leucine and phenylalanine, normally orient inwardly, away from water or polar solutes.
Polar or ionized R-groups, as in glutamine or arginine, orient outwardly to contact the aqueous environment.
Some amino acids, such as glycine, can be accommodated by aqueous or nonaqueous environments.
The rules of solubility and the tendency for secondary structure formation determine how the chain spontaneously folds into its final structure.
- Forces stabilizing protein tertiary structure.
- Hydrophobic interactions — the tendency of nonpolar groups to cluster together to exclude water.
- Hydrogen bonding, as part of any secondary structure, as well as other hydrogen bonds.
- Ionic interactions — attraction between unlike electric charges of ionized R-groups.
- Disulfide bridges between cysteinyl residues.The R-group of cysteine is -CH2-SH.-SH groups can oxidize spontaneously to form disulfides .
R-CH2-SH + R’-CH2-SH + O2 = R-CH2-S-S-CH2-R’ + H2O2
The disulfide bridge is a covalent bond.It strongly links regions of the polypeptide chain that could be distant in the primary sequence.It forms after tertiary folding has occurred, so it stabilizes, but does not determine tertiary structure.
Globular proteins are typically organized into one or more compact patterns called domains.
- Examples
- Triose phosphate isomerase.
- Domain 1 of pyruvate kinase.
- Examples
- Lactate dehydrogenase domain 1
- Phosphoglycerate kinase domain 2
Amino Acids Polymerize To Form Polypeptides Or Proteins
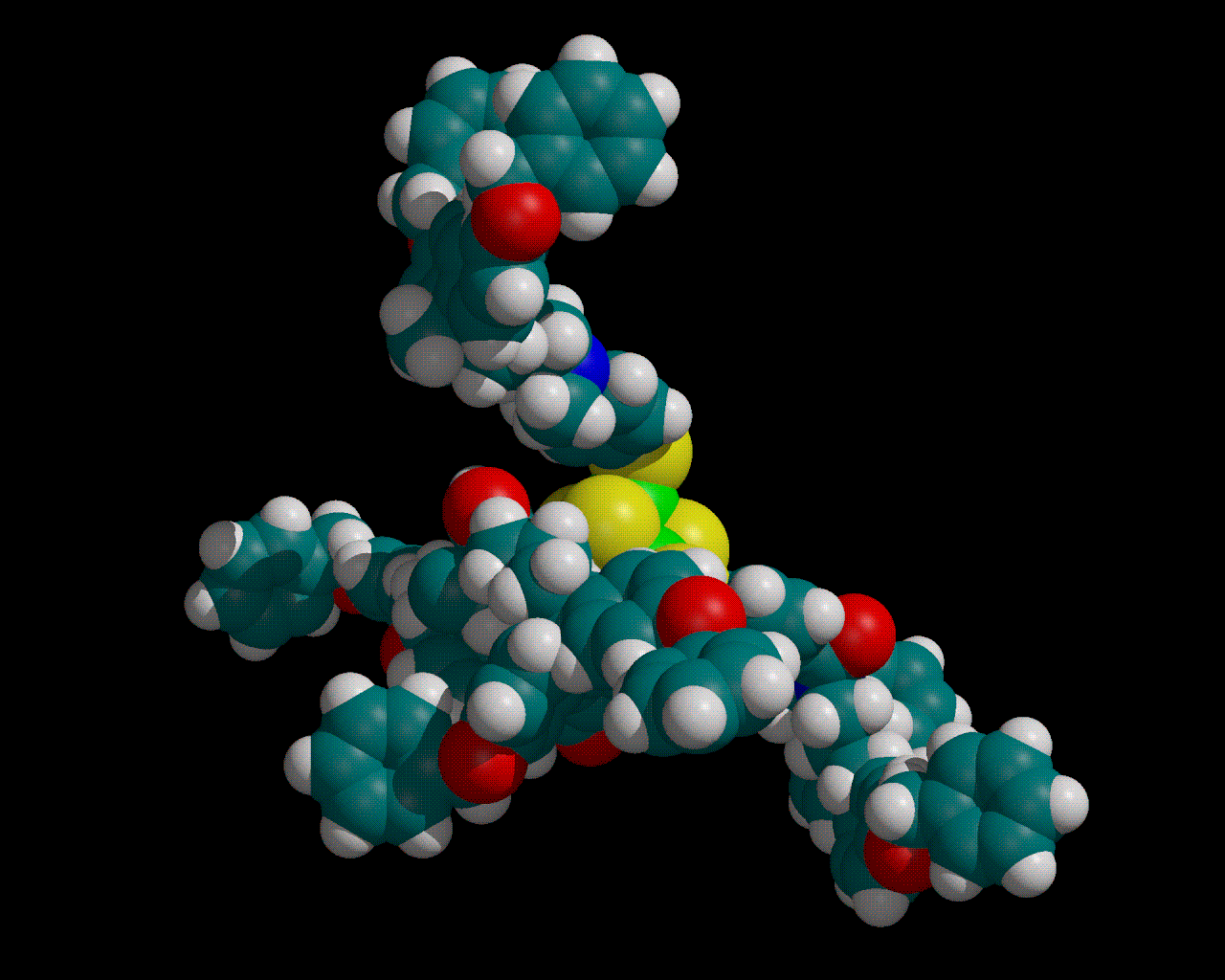
The naturally occurring amino acids are optically active, as they have four different groups attached to one carbon, and have the L-configuration.
The R-groups of the amino acids provide a basis for classifying amino acids. There are many ways of classifying amino acids, but one very useful way is on the basis of how well or poorly the R-group interacts with water
Also Check: Broward County Public Schools Algebra 1 Countdown Answers
Connect These Two Boxes By Circling The Correct Arrow
Biology macromolecules worksheet answers. Inorganic compounds that do not contain both carbon and hydrogen. The benedict s test is used to determine the presence of single sugar carbohydrates. Macromolecule model activity answer key displaying top 8 worksheets found for this concept.
Compounds can be organic or inorganic. Organic compounds that contain both carbon and hydrogen atoms. Start studying macromolecules review worksheet for h biology.
Organic molecules worksheet review answers macromolecule worksheet answer key and carbohydrates worksheet answers are three of main things we will present to you based on the post title. When we talk related with biology macromolecules worksheets and answers we have collected various similar photos to complete your references. Macromolecule review worksheet.
Some of the worksheets for this concept are building macromolecules activity answer key organic macromolecules answer key organic macromolecules answer key biology macromolecules work and answers organic macromolecules work answers organic macromolecules work answers organic macromolecules work. We additionally manage to pay for variant types and as a consequence type of the books to browse. The color blue indicates that no single sugar carbohydrates are present.
Write beneath the line how these two are connected. Answer the following questions. Classes of organic compounds that are central to life on earth.
Pin On Biology S1
Pin On Macromolecules
Mechanisms Of Primary Macromolecule Secretion
The protein subcomponent of saliva is derived primarily from secretory granules of the acinar and ductal cells. Secretory proteins are discharged into the lumen of the secretory unit by a process of exocytosis, wherein fusion of secretory granules with a delimited portion of the plasmalemma of the apical membrane occurs. The membrane fusion is the last of a series of steps required for the transfer of export proteins from their synthesis in the rough endoplasmic reticulum to the extracellular environment. According to the model offered by Palade,11 the secretory process can be divided into six steps: synthesis, segregation, intracellular transport, concentration, intracellular storage, and discharge.
Jaison Jeevanandam, … Michael K. Danquah, in, 2022
Read Also: Algebra With Pizzazz Answer Key Page 34 Books Never Written
Biological Macromolecules Are Polar
The main point of the first segment of this material is this: THE MONOMER UNITS OF BIOLOGICAL MACROMOLECULES HAVE HEADS AND TAILS. WHEN THEY POLYMERIZE IN A HEAD-TO-TAIL FASHION, THE RESULTING POLYMERS ALSO HAVE HEADS AND TAILS.
These macromolecules are polar because they are formed by head to tail condensation of polar monomers. Let’s look at the three major classes of macromolecules to see how this works, and let’s begin with carbohydrates.
Industrial Applications Of Macromolecules
There are three major groups of macromolecules that are essential in the industry, apart from biological macromolecules. These include plastics, fibres, and elastomers.
Elastomers are macromolecules that are flexible and stretchy. The elastic property lets these materials to be used in products like hair bands and elastic waistbands. These objects could be stretched, and they return to their original structure once released.
We wear fibre macromolecules. Nylon, Polyester, and acrylic fibres are used in everything from blouses, belts to shirts and shoes. Natural fibres include wool, cotton, and silk.
Hence, there are many objects that we use today are made up of macromolecules. Many types of plastics are made through a process known as polymerization, which is the joining of monomer units from plastic products.
To know more about macromolecules, its definition, types of macromolecules and its examples, keep visiting BYJUS website or download BYJUS app for further reference.
Also Check: Compass Rose For Kids
Amino Acids Polymerize By Eliminating The Elements Of Water
The product has ends with different properties.
- An end with a free amino group this is called the amino terminal or N-terminal.
- An end with a free carboxyl group this is called the carboxyl terminal or C-terminal.
Conventions for writing sequences of amino acids.
Abbreviations for the amino acids are usually used most of the three letter abbreviations are self-evident, such as gly for glycine, asp for aspartate, etc.
There is also a one-letter abbreviation system it is becoming more common. Many of the one-letter abbreviations are straightforward, for example:
- G = glycine
Others require a little imagination to justify:
- F = phenylalanine .
- Y = tyrosine .
- D = aspartate .
Still others are rather difficult to justify:
- W = tryptophan .
- K = lysine
Question: What do you suppose “Q” represents?
You should be aware this is becoming more and more commonly used, and you should have the mindset of picking it up as you are exposed to it, rather than resisting.
Sequences are written with the N-terminal to the left and the C-terminal to the right.
Although R-groups of some amino acids contain amino and carboxyl groups, branched polypeptides or proteins do not occur.
The sequence of monomer units in a macromolecule is called the PRIMARY STRUCTURE of that macromolecule. Each specific macromolecule has a unique primary structure.
THE REGULAR REPEAT OF MONOMER UNITS HAVING THE SAME SIZE AND THE SAME BOND ANGLES LEADS TO HELICAL POLYMERS.
Nucleotides Polymerize By Eliminating The Elements Of Water
A 3′-> 5′ phosphodiester bond is thereby formed.The product has ends with different properties.
- An end with a free 5′ group this is called the 5′ end.
- An end with a free 3′ group this is called the 3′ end.
Let’s look at the conventions for writing sequences of nucleotides in nucleic acids.Bases are abbreviated by their initials: A, C, G and U or T.U is normally found only in RNA, and T is normally found only in DNA.So the presence of U vs. T distinguishes between RNA and DNA in a written sequence.
Sequences are written with the 5′ end to the left and the 3′ end to the right unless specifically designated otherwise.
Phosphate groups are usually not shown unless the writer wants to draw attention to them. The following representations are all equivalent.
uracil adenine cytosine guanine | | | | P-ribose-P-ribose-P-ribose-P-ribose-OH 5' 3' 5' 3' 5' 3' 5' 3'pUpApCpGUACG3' GCAU 5'
Branches are possible in RNA but not in DNA.RNA has a 2′ -OH, at which branching could occur, while DNA does not.Branching is very unusual it is known to occur only during RNA modification , but not in any finished RNA species.
Recommended Reading: Monitoring Progress And Modeling With Mathematics Geometry Answers
Incorporation Of Nonprotein Components Into Proteins
Many different kinds of compound are found in conjugated proteins. A few examples are:
Nomenclature: the word “conjugated” is from the Latin, cum = with and jugum = yoke. The protein and nonprotein moieties are yoked with one another to work together.
- The apoprotein = the protein without its nonprotein component.
- The prosthetic group = the nonprotein portion alone.
- The conjugated protein = the apoprotein + prosthetic group.
Metalloproteins
- Fe = Fe + e-
- Roles involving simple binding. include
- Complexing several groups of the protein simultaneously, thereby stabilizing the three-dimensional structure of the protein.The protein acts as a polydentate ligand.Example: thermolysin loses its structure if Ca is removed.
- Binding the protein and some other molecule together .
- Participation in the protein’s function. such asactivation of a substrate. When a metal accepts an electron pair form a bound substrate, the resulting electron deficiency may make the substrate more reactive.
- Metals frequently participate in oxidation-reduction. Sometimes bound metals participate directly in biological oxidation-reduction reactions by accepting or donating an electron .
Lipoproteins
What Is Osmotic Pressure
Osmotic pressure is defined as the minimum pressure applied to a solution to stop the flow of solvent molecules through a semipermeable membrane. The osmotic pressure of a solution is proportional to the molar concentration of the solute particles in the solution.
= iCRT is the formula used for finding the osmotic pressure of a given solution.
Recommended Reading: Eoc Fsa Practice Test Algebra 1 Calculator Portion
What Are The Four Types Of Polysaccharides
5. Functions of protein, structure of amino acids, 4 protein structures Structure of amino acid drawn on back of sg 4. Protein structures:
o Primary- chain of sequence of amino acid
o Secondary- alpha helical shape, B beta pleated states o Tertiary – 1 polypeptide
o Quaternary- 2 polypeptide
*Proteins shape determines function * denaturation can result from
6. Structure of nucleotide
o At least one phosphate functional group PO4^3-
o A pentose sugar Don’t forget about the age old question of What is the psychological triad?
o A nitrogenous base RNA VS DNA
DNA- 2 strand, deoxyribose, ATGC,
RNA 1 strand, ribose, AUGC
Notes from ppt ch. 5
Irreducible complexity:
1. Bacteria flagellum If you want to learn more check out What is the meaning of a single unit torso?
2. Chaperonins
3 common features of all cells: plasma membrane, DNA, storing region and cytoplasm
1. Plasma Membrane-
2. DNA storing region in eukaryotic Nucleus nucleolus is in nucleus functions to synthesize RNA nucleus largest organelle in prokaryotic If you want to learn more check out What are the four economic policy criteria?If you want to learn more check out What are the types of marketing information?
cytoplasm- made of cytosol holds all subcellular components
Membrane proteins cell theory
o 1. transport protein: 1. Chanel protein- internal hydrophilic channels 2. Carrier transport protein
o 2. receptor protein
o 3. Recognition protein- recognize molecules/ ions = identification tag
number
Structure And Function Are Related
Macromolecules interact with other molecules using a variety of non-covalent interactions. The specificity and affinity of these interactions are critical to biological function. Some macromolecules catalyze chemical reactions or facilitate physical processes , allowing them to proceed in ambient conditions. These processes can be quantitatively described by rate laws and thermodynamic principles, .
Associated learning goals
- Students should be able to use mechanistic reasoning to explain how an enzyme or ribozyme catalyzes a particular reaction. A
- Students should be able to discuss the basis for various types of enzyme mechanisms. A
- Students should be able to calculate enzymatic rates and compare these rates and relate these rates back to cellular or organismal homeostasis. B
- Students should be able to discuss various methods that can be used to determine affinity and stoichiometry of a ligand-macromolecule complex and relate the results to both thermodynamic and kinetic data. B
- Students should be able to critically assess contributions to specificity in a ligand-macromolecule complex and design experiments to both assess contributions to specificity and test hypotheses about ligand specificity in a complex. C
- Students should be able to predict the biological and chemical effects of either mutation or ligand structural change on the affinity of binding and design appropriate experiments to test their predictions. C
Also Check: Homework 4 Angle Addition Postulate Answers
Examples Of Macromolecule In A Sentence
macromolecules Science | AAASmacromoleculesWiredmacromoleculesWiredmacromoleculesWiredmacromoleculesWiredmacromoleculesWiredmacromoleculesWiredmacromolecules Popular Mechanics
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘macromolecule.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
What Are Four Major Macromolecules And What Is Their Structure And Function
The four macromolecules are nucleic acids, carbohydrates, proteins, and lipids.
Explanation:
1. Nucleic acids: Contain N in rings, nucleotides made of sugar, phosphate and nitrogenous base
Carbohydrates: Made of C,H, and O OH’s on all carbons except one
Lipid: Made of C,H, and O lots of C-H bonds may have some C=C bonds
Protein: Contain N, have N-C-C backbone
Function:
Also Check: Paris Jackson Father
Nucleotides Polymerize To Yield Nucleic Acids
There are four dominant bases here are three of them:
The fourth base is
- uracil or
- thymine
Be aware that uracil and thymine are very similar they differ only by a methyl group.
You need to know which are purines and which are pyrimidines, and whether it is the purines or the pyrimidines that have one ring. The reasons for knowing these points relate to the way purines and pyrimidines interact in nucleic acids, which we’ll cover shortly.
What Do Elements And Mixtures Have In Common
Elements and compounds are purely homogeneous substances and they have a constant composition throughout. … A compound can be broken down into its constituents. Some examples of compounds are sodium chloride , water, etc. A mixture is composed of two or more elements or compounds in a non-fixed ratio.
Also Check: The Branch Of Chemistry That Involves The Study Of Substances
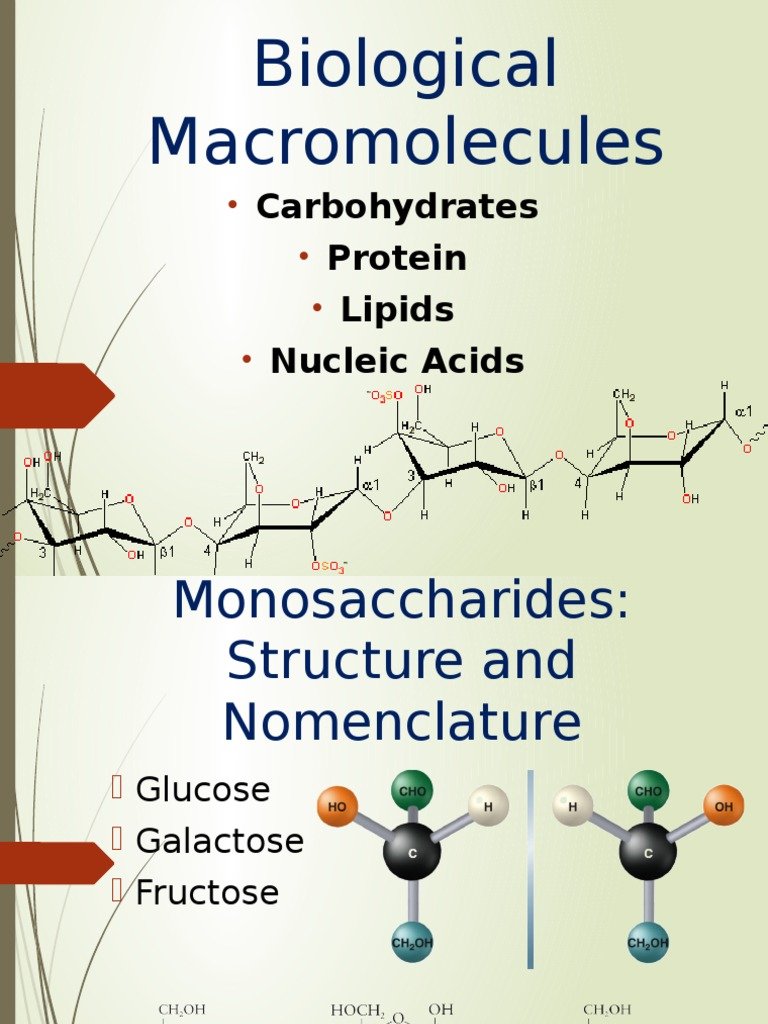
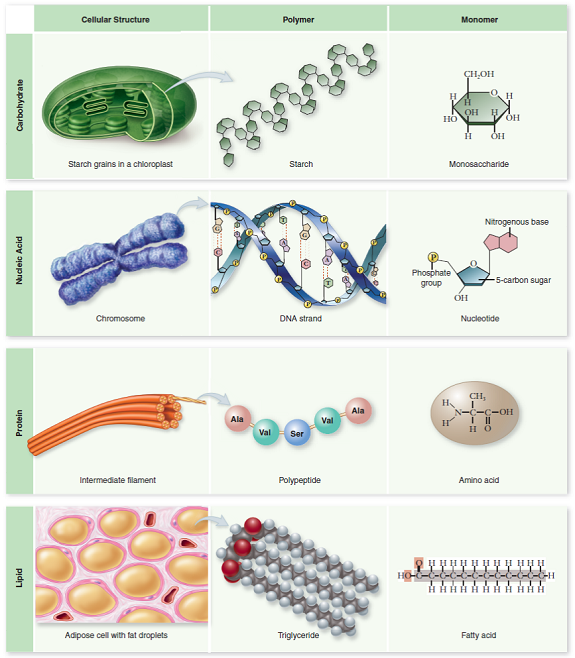
Four Classes Of Biological Macromolecules
There are four major classes of biological macromolecules:
Each of these types of macromolecules performs a wide array of important functions within the cell a cell cannot perform its role within the body without many different types of these crucial molecules. In combination, these biological macromolecules make up the majority of a cells dry mass. All the molecules both inside and outside of cells are situated in a water-based environment, and all the reactions of biological systems are occurring in that same environment.
Interactive: Monomers and Polymers
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
Boundless.
A Variety Of Experimental And Computational Approaches Can Be Used To Observe And Quantitatively Measure The Structure Dynamics And Function Of Biological Macromolecules
A variety of experimental and computational approaches can be used to observe and quantitatively measure the structure, dynamics and function of biological macromolecules. Equations can be derived from models and used to predict outcomes or analyze data. Data can be analyzed statistically to assess the correctness of the model and the reliability of the data.
Associated learning goals
- Students should be able to propose a purification scheme for a particular molecule in a mixture given the biophysical properties of the various molecules in the mix. B
- Students should be able to either propose experiments that would determine the quaternary structure of a molecule or be able to interpret data pertaining to tertiary and quaternary structure of molecules. B
- Students should be able to explain how computational approaches can be used to explore protein-ligand interactions and discuss how the results of such computations can be explored experimentally. C
- Students should be able to compare and contrast the computational approaches available to propose a three dimensional structure of a macromolecule and discuss how the proposed structure could be validated experimentally. C
- Students should be able to analyze kinetic or binding data to derive appropriate parameters and asses the validity of the model used to describe the phenomenon. C
You May Like: Who’s Khloe Kardashian’s Real Dad