Molecules With More Than One Central Atom
The VSEPR theory not only applies to one central atom, but it applies to molecules with more than one central atom. We take in account the geometric distribution of the terminal atoms around each central atom. For the final description, we combine the separate description of each atom. In other words, we take long chain molecules and break it down into pieces. Each piece will form a particular shape. Follow the example provided below:
Butane is C4H10. C-C-C-C is the simplified structural formula where the Hydrogens are implied to have single bonds to Carbon. You can view a better structural formula of butane at en.Wikipedia.org/wiki/File:Butane-2D-flat.png If we break down each Carbon, the central atoms, into pieces, we can determine the relative shape of each section. Let’s start with the leftmost side. We see that C has three single bonds to 2 Hydrogens and one single bond to Carbon. That means that we have 4 electron groups. By checking the geometry of molecules chart above, we have a tetrahedral shape. Now, we move on to the next Carbon. This Carbon has 2 single bonds to 2 Carbons and 2 single bonds to 2 Hydrogens. Again, we have 4 electron groups which result in a tetrahedral. Continuing this trend, we have another tetrahedral with single bonds attached to Hydrogen and Carbon atoms. As for the rightmost Carbon, we also have a tetrahedral where Carbon binds with one Carbon and 3 Hydrogens.
How Many Pairs Of Electrons Are In Chloride
Explanation: An isolated chlorine ATOM has 7 valence electrons. And thus the parent dichlorine molecule tends to be an OXIDANT, viz And because the resultant chloride anion has FOUR SUCH LONE PAIRS, instead of the SEVEN electrons associated with the NEUTRAL atom, chloride has a FORMAL NEGATIVE CHARGE.
Ab4e: Sulfur Tetrafluoride Sf4
The Lewis structure for SF 4 contains four single bonds and a lone pair on the sulfur atom .
Figure 12. Lone pair electrons in SF4.
The sulfur atom has five electron groups around it, which corresponds to the trigonal bipyramidal domain geometry, as in PCl 5 . Recall that the trigonal bipyramidal geometry has three equatorial atoms and two axial atoms attached to the central atom. Because of the greater repulsion of a lone pair, it is one of the equatorial atoms that are replaced by a lone pair. The geometry of the molecule is called a distorted tetrahedron or seesaw.
Figure 13. Ball and stick model for SF4 .
| Total Number of Electron Pairs | Number of Bonding Pairs |
- Electron pairs repel each other and influence bond angles and molecular shape.
- The presence of lone pair electrons influences the three-dimensional shape of the molecule.
Don’t Miss: Cpm Algebra 1 Chapter 9 Answers Pdf
Whats The Polarity Of Nh3
NH3 is polar as a result of it has 3 dipoles that dont cancel out. Every N-H bond is polar as a result of N is extra electronegative than H. NH3 is general asymmetrical in its VSEPR form, so the dipoles do not cancel out and its due to this fact polar. Nov 26, 2017
Why does ammonia have a tetrahedral electron geometry however a trigonal pyramidal molecular geometry?
Ammonia additionally has 4 electron pairs and the geometry of nitrogen relies upon a tetrahedral association of electron pairs. There are simply three bonded teams, due to this fact there may be one lone pair. Nonetheless because the lone pairs are invisible, the form of ammonia is trigonal pyramidal.
What number of electron domains does PCl3?
All in all, if we have a look at PCl3, there are 3 electron pairs, 3 bond pairs and one lone pair of electrons.
What number of lone pairs does f2 have?
three lone pairs In fluorine molecule, the 2 fluorine atoms every donate one electron to the bond after which share that bonding pair. every of the atoms then has 6 valence electrons not concerned within the bond, so every fluorine atom has three lone pairs of electrons on it.
Why does water have 2 lone pairs?
How Does An Electroscope Work
An electroscope is a device used to study charge. When a positively charged object nears the upper post, electrons flow to the top of the jar leaving the two gold leaves postivley charged. The leaves repel each other since both hold postive, like charges. The VSEPR theory says that electron pairs, also a set of like charges, will repel each other such that the shape of the molecule will adjust so that the valence electron-pairs stay as far apart from each other as possible.
Don’t Miss: Unit 1 Test Study Guide Geometry Basics Gina Wilson
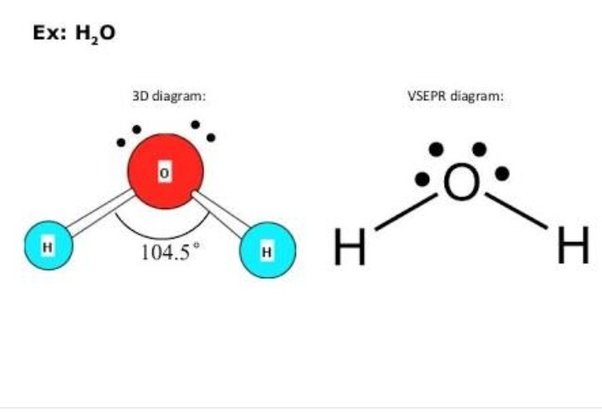
Understanding The Electronic Geometry Of H2o
The H2O molecule is composed of two hydrogen atoms and one oxygen atom. It forms a bond angle of 104.5°. As a result, it is feasible to determine that it is bent in the form of an H2O molecule.
According to Lewiss structure, a lone pair exists when all of the atoms valence electrons are unpaired.
The H2O molecules Lewis structure reveals two solitary sigma bonds between the O and H. Additionally, and these connections leave two lone pairs of electrons on the oxygen atom, which adds significantly to the H2O molecules tetrahedral bent geometrical configuration.
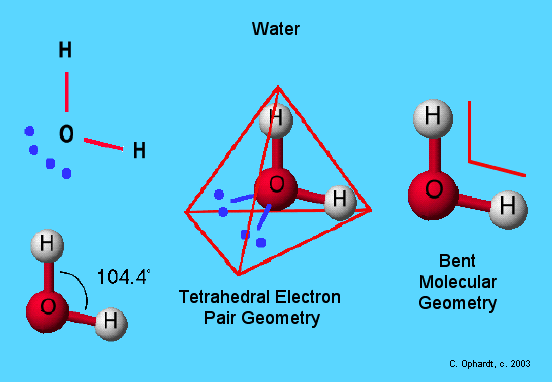
What Is The Electron Pair Geometry Of H2o
The electron pair geometry of water, with the chemical formula H2O, is a tetrahedral. This structure gives a water molecule a bent molecular shape.
A molecule is the smallest fundamental unit of a pure chemical compound. It comprises two or several types of atoms. A water molecule is one of the most commonly occurring molecules in nature. It consists of two hydrogen atoms that are chemically bonded to a single oxygen atom.
A water molecule has two electron pairs and two bond pairs. In a molecule, the bond pairs and lone pairs are positioned in a manner that results in the least amount of repulsion between them, based on the valence shell electron pair repulsion, or VSEPR, theory. The tetrahedral electron pair geometry of a water molecule results in its angular form, where the bond angle between the atoms is 104.5 degrees.
Read Also: Homework 5 Angle Addition Postulate Answer Key
The Molecular Geometrical Of The H2o Lewis Structure For The Molecular Cluster
The bond angle between helium, oxygen, and hydrogen atoms is 104.5°. The geometrical configuration of a single H2O molecule is twisted, as can be seen from this. The water molecule is nonlinear, with an above said angle.
Its demonstrated by the Valence Shell Electron Pair Repulsion principle, which explains why the bond angle on the oxygen atom is limited to 104.5° despite the presence of two pairs of lone electrons.
A bent-shaped molecules optimal bond angle is 109.5°. Because each O-H bond are polar covalent in nature, with more positive end at H as compare with O end. This induce H2O molecule higher dipole moment.
When all of the valence electrons around the atom are not paired, a lone pair occurs, according to the Lewis structure.
The oxygen atom in the H2O molecule, which has two lone pairs, is similar.
Due to the lone pair-lone pair repulsion, which is greater than the bond pair-bond pair and lone pair-bond pair repulsion, these lone pairs skew the bond angle.The bond angle reduces as the lone pair increases. Since the oxygen atom has two lone pairs, the bond angle is reduced to 104.5°.
Central Atom With One Or More Lone Pairs
The molecular geometries of molecules change when the central atom has one or more lone pairs of electrons. The total number of electron pairs, both bonding pairs and lone pairs, leads to what is called the electron domain geometry. When one or more of the bonding pairs of electrons is replaced with a lone pair, the molecular geometry of the molecule is altered. In keeping with the A and B symbols established in the previous section, we will use E to represent a lone pair on the central atom . A subscript will be used when there is more than one lone pair. Lone pairs on the surrounding atoms do not affect the geometry.
You May Like: My.hrw Answers
Molecular Orbital Diagram Of Water
The molecular orbital diagram is a pictorial representation of determining chemical bonding between the molecules of a compound.
Furthermore, the molecular orbital diagram helps with determining how two sigma bonds have been formed and the effect of the lone pairs on the structure.
From the above diagram, it can be seen that the six valence electrons are bonding with the 1s orbital electrons of the hydrogen atom.
The mixing and overlapping are occurring among the atomic orbital of similar energy.
It is taking place in such a manner that the bonding electrons in lower energy are forming antibonding molecular orbitals of higher energy.
The left oxygen electrons do not overlap further due to the scarcity of electrons.
The oxygen atom has its electronegativity higher than hydrogen. Due to this, oxygen has a higher negative charge, whereas hydrogen has a positive charge. It makes oxygen attract nearby electrons and form a bond ultimately.
On the other hand, the hydrogen does not react with nearby molecules as it has already fulfilled its orbital and bonded with oxygen through a sigma bond, which is not easy to break.
It leads to the formation of polarity in an H2O molecule, irrespective of having a net neutral charge.
You can also check an interesting article written about the polarity in water.
What Was The Molecular Geometry For Scl6
The electron pair geometry and molecular geometry are both . All bond angles are 90. An example is sulfur hexachloride .
You May Like: Coolmath.coms
H2o Lewis Structure And Molecular Geometry
Water is very well known molecular species in earth. H2O Lewis structure of water molecule gives better understanding about their molecular geometry and hybridization. The most important oxide of hydrogen is H2O.
Water, one of the Earths largest components, has the molecular formula H2O. Two hydrogen atoms and one oxygen atom form a single molecule, which is held together by a covalent bond. Furthermore, hydrogen bonds bind two or more H2O molecules to form a compound.
Its worth noting that covalent bonds are stronger than hydrogen bonds, which is why water reacts readily with the majority of chemical elements on the periodic table. Water having hydrogen bonding property.
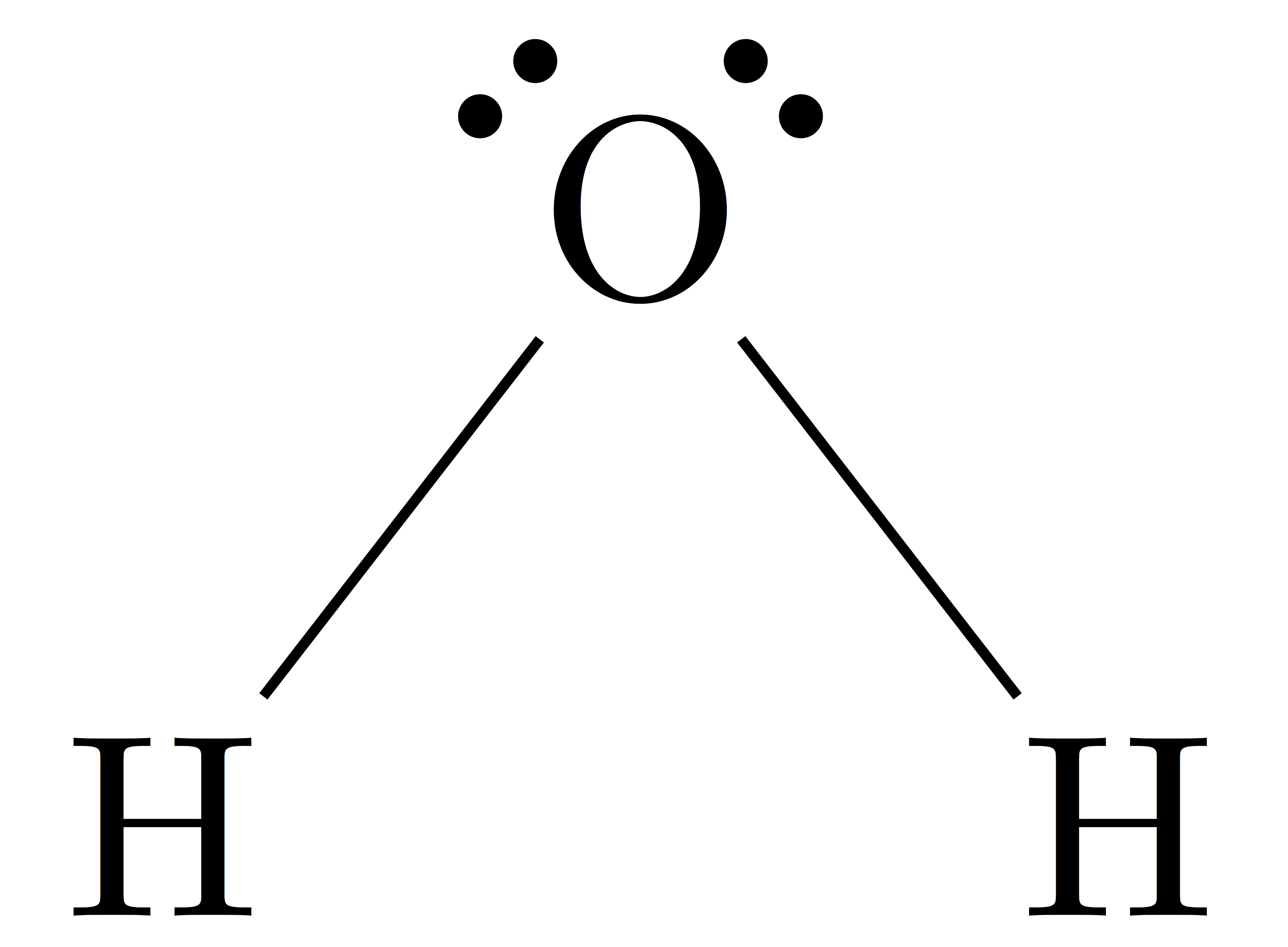
The Lewis structure, also known as the electron dot structure, is dotted diagrammatic description of calculating the total number of valence electrons present in an atom that are ready to form bonds to form a molecule and, eventually, a compound.
The valence electrons are shown as dots surrounding the atom sign, usually in pairs all four direction.
A hydrogen atoms atomic number is one, so its electronic structure is 1s1. There is a shortage of one more electron since the 1s shell will only hold two electrons.
What Are The Valence Electrons Of Hydrogen And Oxygen
The valence electrons are the free electrons in the atoms outermost casing. Since it is the farthest out, the nucleus retains the outer shell with a shaky grip.
In addition, unpaired valence electrons become extremely reactive in nature, taking or contributing electrons to maintain the outermost shell.
Its worth noting that the greater the number of valence electrons, the more powerful the ability to accept electrons.The atoms capacity to donate valence electrons increases as the number of valence electrons decreases.
Read Also: Geometry Segment And Angle Addition Worksheet Answers
What Is The Electronic Geometry Of H2o Enter The Electronic Geometry Of The Molecule
H2O Molecular Geometry, Lewis Structure, Shape and Bond Angles
| Name of molecule | |
| No of Valence Electrons in the molecule | 8 |
| Molecular Geometry of H2O | Bent |
What is the molecular geometry of h2o enter the molecular geometry of the molecule?, Thus, the molecular geometry of H2O is bent.
Furthermore, What is molecular geometry of water?, The molecular geometry of the water molecule is bent. The H-O-H bond angle is 104.5°, which is smaller than the bond angle in NH3 . Figure 11. Water molecule.
Finally, What is the electron domain geometry EDG of h2o?, H2O Water:
Electron geometry: tetrahedral. Hybridization: sp3. Then draw the 3D molecular structure using VSEPR rules: Decision: The molecular geometry of H2O is bent with asymmetric charge distribution about the central oxygen atom.
What Is The Electronic Geometry Of H2o
-
The electron pair geometry is tetrahedral.
The molecule itself is bent shape with a 109 degree bond angle.
This is because the orbitals are sp3 hybridized and there are 2 bonding electron pairs and 2 lone pairs.
-
H2o Electron Geometry
-
what is the electronic geometry of H2O?
-
For the best answers, search on this site shorturl.im/IHuiP
HNO2: trigonal planar and bent CH3COOH trigonal planar for both H2O tetrahedral and bent CO linear CH3CH2OH tetrahedral for both at carbon CH4 tetrahedral for both HNO3 trigonal planar for both CO2 linear for both
-
Based on VSEPR Theory the electron clouds and lone pair electron around the O will repel each other.
-
j129 is right, it is a Tetrahedral, but the bond angle is 104.5, not 109 degrees.
-
H2O .
H…….H
Don’t Miss: Ccl4 Molecular Shape
What Is The Octet Rule Law
The states that an atom should have a maximum of eight valence electrons. Furthermore, in the Lewis structure, these eight electrons are drawn just around the atoms sign.
Two valence electrons are of short supply in oxygen. The two hydrogen atoms, on the other hand, have a limit of two valence electrons missing.
The Lewis structure of H2O is drawn in such a way that each atoms deficiency is satisfied.
How Do You Draw Lewis Structures
How to Draw a Lewis Structure
Read Also: Glencoe Geometry Worksheets
Why The Molecular Geometry Of H2s Is Bent Whereas Its Electron Geometry Is Tetrahedral
As we already discussed, electron geometry is determined with the help of both lone electron pairs and bonds pair in a molecule whereas molecular geometry determined using only the bonds present in the molecule.
According to the H2S lewis structure, the central atom has 2 lone pairs and 2 bonded pairs, hence, according to the VSEPR theory, H2S has 4 regions of density around the central atom.
Four regions of electron density always form a tetrahedral geometry, hence, the electron geometry of H2S is tetrahedral.
While calculating the molecular shape, we will not consider the lone pair as the molecular shape has to do with the shape of the actual molecule, not the electrons, so they are not accounted for in that sense.
But we cant neglect the effect of lone pair on a bond angle while calculating the molecular shape of the molecule.
As there are two lone pairs present on the sulfur central atom in the H2S molecule, hence, it will contract the bond pair, and this makes its shape appears like a bent structure.
Therefore, the molecular geometry or shape of H2S is bent while its electron geometry is tetrahedral.
Hybridization Of H2o Molecule
The bond between each oxygen and hydrogen atom in a water molecule is sigma with no pi bonds.
As we know, sigma bonds are the strongest covalent bonds. As a result, there is high stability between the oxygen and the hydrogen atom.
It is the two lone pairs on the oxygen atom which makes all the difference. The hybridization of a water molecule is sp3, where its oxygen has been hybridized.
According to the diagram, it can be analyzed that the single oxygen atom in the water molecule has one 2s orbital and three 2p orbitals. These four altogether leads to the formation of four sp3 hybridized orbitals.
It leads to the formation of the tetrahedral bent geometry, where overall H2O molecule shows 25% characteristics of s and 75% characteristics of the p orbital.
It can further be explained with the help of a molecular orbital diagram of the H2O molecule.
The 2s orbital and three 2p orbitals of the oxygen atom forms four new hybrid orbitals which further bonds by undergoing overlapping with the 1s orbital of the hydrogen atoms.
Read Also: Edgenuity Algebra 1 Answers
What Are The Valence Electrons
The valence electrons are free electrons present in the outermost shell of the atom. The nucleus holds the outer shell weakly as it is farthest in the distance.
Moreover, if the valence electrons are unpaired, they become highly reactive in nature by either accepting or donating electrons to stabilize its outermost shell.
It is interesting to realize that the larger the number of valence electrons, the stronger will be the ability to accept the electrons.
Whereas, the smaller the number of valence electrons, the stronger will be the ability of the atom to donate them.
What Number Of Electron Domains Are In H2o
5 electron domains Desk of Three to Six Electron Domains Electron Domains Association of Electron Domains Examples H2O, SCl2 5 Trigonal bipyramidal PCl5, AsF5 SF4 ClF3 9 extra rows
Is NH3 bent or linear?
If these are all bond pairs the molecular geometry is tetrahedral . If there may be one lone pair of electrons and three bond pairs the ensuing molecular geometry is trigonal pyramidal . If there are two bond pairs and two lone pairs of electrons the molecular geometry is angular or bent .
What number of domains does ammonia have?
4 Within the case of NH3 there are 4 electron domains across the nitrogen atom . The repulsions amongst 4 electron domains are minimized when the domains level towards the vertices of a tetrahedron . The tetrahedral association of the electron domains in ammonia is proven in Determine 9.5.
Whats the distinction between electron area geometry and molecular geometry?
Re: Distinction between molecular and electron geometry? Electron geometry describes the association of electron teams. Molecular geometry describes the association of atoms, excluding lone pairs. For instance, within the case of a trigonal planar form as outlined by electron geometry, there are three bonds. Nov 27, 2017
Read Also: Chapter 2 Test Form 2c