Which Technique Provides Better Candidates
The ISE ligand based model achieved a better result than the pharmacophore model. Based on our kinetic measurements and supported by docking, inhibitor T5450157, a result of ISE, fills the S4-S5 subpockets while inhibitor T6816369, based on the pharmacophore model, resides beyond the S4-S5 pockets. Another difference between ISE and pharmacophore models is in their scoring of the crystallographic original inhibitors compared to the scoring of fragments in both. The original inhibitors are not expected to score well compared to the fragments which are the basis of the models. S6 Table presents the difference in results between the full inhibitors and the fragments, in their MBI scores of ISE. In contrast, as mentioned above, we have not found any difference between these sets according to the pharmacophore model. A possible explanation for the relative success of ISE compared to pharmacophore is that the latter is based on a crystal structure and is more “rigid” while the ISE model is based on a large set of fragments from structures that could attain different conformations. In that case, the ISE model allows more flexibility around the binding site.
Enzyme Inhibition And Types Of
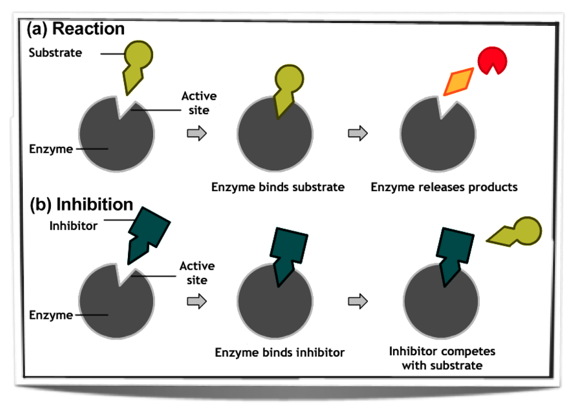
Posted: Aug 20, 2020 · The enzyme inhibition in which the enzymatic activity can be regained after removal of inhibitors. Types of reversible inhibition: i). Competitive inhibition. Competitive inhibitors are substrate analog that bind to substrate binding site of enzyme i.e. active site so competition occurs between inhibitor and substrate for binding to enzyme.
Does The Zone Of Inhibition Edge Indicate
For best results enter two or more search terms.MICRO.
| Question | Answer |
|---|---|
| does the zone of inhibitions edge indicate the limit of antibacterial agent diffusion into the agar? | No. edge is no the unit of diffusion. diffusion occurs beyond edge, but concentration of Bactraicin is to dilute to be lethal -the edge represent MIC |
Recommended Reading: What Math Is Needed For Finance
Don’t Miss: Geometry Dash Hack No Root
Enzyme Inhibitors As Metabolic Poisons
-
Many poisons work by inhibiting the action of enzymes involved in Metabolic processes, which disturbs an organism.
-
For example, Potassium Cyanide is an irreversible Inhibitor of the enzyme Cytochrome C Oxidase, which takes part in respiration reactions in cells. If this enzyme is inhibited, ATP cannot be made since Oxygen use is decreased. This means that cells can only respire Anaerobically, leading to a build up of Lactic Acid in the blood. This is potentially fatal.
-
The poison Malonate binds to the Active Site of the enzyme Succinate Dehydrogenase competing with Succinate, which is important in respiration.
Evidence For The Physical Continuity Of Soluble And Nerve Ending Tryptophan Hydroxylase
Additional evidence was sought for the cell body location of the soluble enzyme, for the nerve ending location of the particulate enzyme, and the relationship between them. This was done using the strategy of the acute administration of the tryptophan hydroxylase inhibitor parachlorophenylalanine and following the flow of reduced activity from the midbrain region to the septal areas. Due to the multiplicity of actions of PCPA, both in vitro and in vivo experiments were carried out. Figure 3 demonstrates that PCPA is a competitive inhibitor of uptake of labeled tryptophan into synaptosomes with a Ki of 9.7 Ã 106 M. Other work has demonstrated a dialyzable competitive and non-dialyzable non-competitive inhibition of soluble enzyme by PCPA several hours after administration . In a series of in vivo experiments, PCPA was administered and groups of animals were sacrificed after varying periods of time. Tryptophan hydroxylase activity was determined in the midbrain and septal areas. Figure 4 summarizes the results. The midbrain manifests a decreased activity during the first few hours and a delayed decrease in two days which returned to control levels between eight and thirteen days after drug treatment. The septal area manifested a more immediate decrease, reversible in four hours, and a delayed decrease reaching the septal area in eight to thirteen days after drug administration.
Ivar Roots, ⦠Santosh Nigam, in, 1980
Read Also: Different Fields Of Chemistry
What Is Inhibition In Biology
4.8/5biologyinhibitbiological inhibitorinhibitorbiologicalexplained here
Inhibit comes from the Latin inhibitus, meaning “to hold in”, “to restrain”, or “to keep”. In biology, there are various molecules whose function is to inhibit. In biology, an inhibiting molecule controls, prevents, restrains, arrests, or regulates, as in “to inhibit an action”.
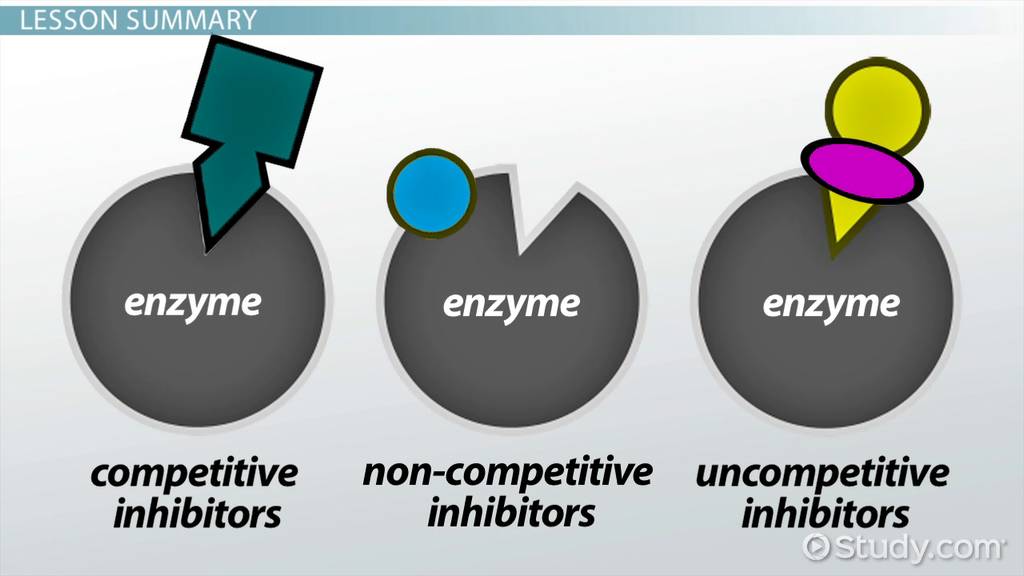
Furthermore, what are the three types of enzyme inhibition? We will discuss four types of enzyme inhibition competitive, non- competitive, uncompetitive, and suicide. Of these, the first three types are reversible.
Subsequently, question is, what does an inhibitor do?
Enzyme inhibitors are molecules or compounds that bind to enzymes and result in a decrease in their activity. An inhibitor can bind to an enzyme and stop a substrate from entering the enzyme’s active site and/or prevent the enzyme from catalyzing a chemical reaction. There are two categories of inhibitors.
What is a non competitive inhibitor in biology?
Non–competitive inhibition is a type of enzyme inhibition where the inhibitor reduces the activity of the enzyme and binds equally well to the enzyme whether or not it has already bound the substrate.
Quantitative Description Of Reversible Inhibitors
Most reversible inhibitors follow the classic Michaelis-Menten scheme, where an enzyme binds to its substrate to form an enzyme-substrate complex . km is the Michaelis constant that corresponds to the concentration of the substrate when the velocity is half the maximum. Vmax is the maximum velocity of the enzyme.
- Competitive inhibitors can only bind to E and not to ES. They increase Km by interfering with the binding of the substrate, but they do not affect Vmax because the inhibitor does not change the catalysis in ES because it cannot bind to ES.
-
Diagram showing competitive inhibition
-
Competitive inhibition can also be allosteric, as long as the inhibitor and the substrate cannot bind the enzyme at the same time
-
Another possible mechanism for allosteric competitive inhibition
- Uncompetitive inhibitors can only bind to the ES complex. Therefore, these inhibitors decrease Km because of increased binding efficiency and decrease Vmax because they interfere with substrate binding and hamper catalysis in the ES complex.
- Mixed inhibitors can bind to either E or ES complex, but have a preference for one or the other. This can either increase or decrease Km, respectively. Both cause a decrease in Vmax.
- Non-competitive inhibitors have identical affinities for E and ES. They do not change Km, but decreases Vmax.
Don’t Miss: Eoc Fsa Practice Test Algebra 1 No Calculator Portion
Therapeutic Use Of Enzyme Inhibitors
As illustrated in Table 2, enzymes take part in a wide range of human diseases, and many specific enzyme inhibitors have been developed to combat their activities, thus acting as therapeutic agents.
Type of enzyme inhibitor
Table 2 Selected enzymes inhibitors and their pharmaceutical applications.13,14
Antifungal drugs
Figure 4 Pathways of fungal inhibition by enzyme inhibitor.20-22
Figure 5 Molecular docking showing interaction of FMDP inside the glutamine-binding site of GlcN-6-P synthase.18
Cancer therapies drugs
The initiation and progress of human cancer is associated with kinase. The development of protein kinase inhibitors has proven successful in clinical treatment of various types of cancer.2023 Imatinib and dasatinib, kinase inhibitors have proved a significant increase in patient survival incidence in myeloid leukemia and gastrointestinal stromal tumors . They are approved by the FDA. Structural analysis, molecular docking and magnetic resonance spectroscopy ensured the development of specific and robust kinase inhibitors24 such as nilotinib, approved for imatinib-resistant CML and with a selectivity profile like imatinib.25
Enzyme inhibitors and human disease disorders treatment
Examples Of Reversible Inhibitors
As enzymes have evolved to bind their substrates tightly, and most reversible inhibitors bind in the active site of enzymes, it is unsurprising that some of these inhibitors are strikingly similar in structure to the substrates of their targets. Inhibitors of DHFR are prominent examples. Other example of these substrate mimics are the protease inhibitors, a very successful class of antiretroviral drugs used to treat HIV. The structure of ritonavir, a protease inhibitor based on a peptide and containing three peptide bonds, is shown on the right. As this drug resembles the protein that is the substrate of the HIV protease, it competes with this substrate in the enzyme’s active site.
Enzyme inhibitors are often designed to mimic the transition state or intermediate of an enzyme-catalyzed reaction. This ensures that the inhibitor exploits the transition state stabilising effect of the enzyme, resulting in a better binding affinity than substrate-based designs. An example of such a transition state inhibitor is the antiviral drug oseltamivir this drug mimics the planar nature of the ring oxonium ion in the reaction of the viral enzyme neuraminidase.
Also Check: How Old Are Elton Johns Kids
To Your Health: Penicillin
Chemotherapy is the strategic use of chemicals to destroy infectious microorganisms or cancer cells without causing excessive damage to the other, healthy cells of the host. From bacteria to humans, the metabolic pathways of all living organisms are quite similar, so the search for safe and effective chemotherapeutic agents is a formidable task. Many well-established chemotherapeutic drugs function by inhibiting a critical enzyme in the cells of the invading organism.
An antibiotic is a compound that kills bacteria it may come from a natural source such as molds or be synthesized with a structure analogous to a naturally occurring antibacterial compound. Antibiotics constitute no well-defined class of chemically related substances, but many of them work by effectively inhibiting a variety of enzymes essential to bacterial growth.
Penicillin functions by interfering with the synthesis of cell walls of reproducing bacteria. It does so by inhibiting an enzymetranspeptidasethat catalyzes the last step in bacterial cell-wall biosynthesis. The defective walls cause bacterial cells to burst. Human cells are not affected because they have cell membranes, not cell walls.
Some strains of bacteria become resistant to penicillin through a mutation that allows them to synthesize an enzymepenicillinasethat breaks the antibiotic down . To combat these strains, scientists have synthesized penicillin analogs that are not inactivated by penicillinase.
Production Of Amino Acids
The human body uses twenty different amino acids the building blocks of protein. All amino acids share some common features, and some are very similar to each other. This means that different amino acids are made from the same raw materials.
However, the cell may need different amino acids at different times. Some cells need to make large amounts of proteins that consist of just one or two amino acids others may need all of the amino acids, or the same cell may need different amino acids at different times.
This means that just like converting glucose to ATP, cells must find a way to efficiently use their raw materials to make exactly what they need at any given time. And just like with ATP, they use feedback regulation to ensure they produce only the amino acids they need at any given time.
The first unique step in the biochemical pathway for each amino acid called the committed step, because at that point the cell is committed to using the raw material to produce the amino acid is allosterically regulated by the amino acid itself.
So when there is a lot of taurine in a cell that isnt being used, for example, that serine will bind to the first enzyme in the pathway that makes more serine. As a result, more serine will not be made until the cells serine levels drop.
In this way, cells ensure that raw materials are available for making the amino acids they need and that they are not consumed by making amino acids they dont need.
Don’t Miss: The Segment Addition Postulate Answer Key With Work
Function Of Feedback Inhibition
Feedback inhibition allows the body to avoid many potentially dangerous situations, including:
- Waste. Without feedback inhibition, energy or raw materials that could be used for important cellular functions might be wasted on unnecessary ones.
- Prevents depletion. Without feedback inhibition, raw materials and energy might be depleted by biochemical processes that dont stop, even when their end product is not needed. A good example of this is the production of ATP from glucose. The enzymes that produce ATP from glucose are subject to feedback inhibition by ATP. This saves glucose by preventing its unnecessary breakdown when the cell has plenty of ATP.
- Prevents dangerous build-up. The end products of some biochemical pathways can actually be dangerous in high concentrations. Cholesterol is an excellent example of something our body can make that is good in small quantities but dangerous in large quantities.
- Maintain homeostasis. An essential function of life is the ability to maintain constant internal circumstances in the face of changing environmental circumstances. Some chemical messengers that are involved in maintaining homeostasis are regulated through feedback regulation.
Antiviral Activity Of Ac
The human hepatoma cell line Huh7 was maintained in DMEM supplemented with 10% fetal bovine serum , 2% HEPES 1M , 5ml of sodium bicarbonate 7.5% 1% nonessential amino acids and 1% penicillin-streptomycin 10,000Uml1 in a humidified 5% CO2 incubator at 37°C. Assay medium, used for producing virus stocks and antiviral testing, was prepared by supplementing DMEM with 4% FBS, 2% HEPES 1M, 5ml of sodium bicarbonate 7.5 and 1% NEAA.
To quantify antiviral activity on Huh7 cells, we selected a SARS-CoV-2 virus strain that produces sufficient cytopathogenic effect on this cell line. We started from passage 6 of the SARS-CoV-2 strain BetaCov/Belgium/GHB-03021/2020 that has been described previously, and passaged this three additional times on Huh7 cells while selecting those cultures that showed most CPE. This resulted in a virus stock that confers full CPE on Huh7 per ml) as well as on VeroE6 cells . The genotype of this virus stock shows four nucleotide changes as compared with the mother virus stock and these are currently being analyzed. None of the nucleotide changes occur in the part of the genome that encodes the 3C-like protease, validating this virus stock for testing protease inhibitors.
All virus-related work was conducted in the high-containment BSL3+ facilities of the KU Leuven Rega Institute under licenses AMV 30112018 SBB 219 2018 0892 and AMV 23102017 SBB 219 2017 0589 according to institutional guidelines.
You May Like: How To Get Moles Chemistry
Recommended Reading: Exponential Growth And Decay Common Core Algebra 1 Homework Answers
Examples Of Inhibitor In A Sentence
inhibitorThe Conversationinhibitor Forbesinhibitor ForbesinhibitorQuartzinhibitorQuanta Magazineinhibitor Forbesinhibitor Quanta Magazineinhibitor USA TODAY
These example sentences are selected automatically from various online news sources to reflect current usage of the word ‘inhibitor.’ Views expressed in the examples do not represent the opinion of Merriam-Webster or its editors. Send us feedback.
Why Do Susceptibility Breakpoints Differ For Different Antibiotics
These differences may result from different dosing of drugs in various countries or from use of different laboratory methods to determine antibiotic susceptibility. In addition, some philosophical differences may exist among the various organizations and societies responsible for issuing these breakpoints.
Read Also: What Is Cro In Physics
Don’t Miss: Algebra 1 Age Word Problems
What Is The Role Of Competitive Inhibitor
The competitive inhibitor is an enzyme inhibitor, a molecule that binds to an active site of the enzyme and decreases its activity by preventing the binding of substrate to the active site of an enzyme. Because of the presence of the inhibitor, fewer active sites are available to act on the substrate.
Examples Of Allosteric Inhibition
An example of an allosteric inhibitor is ATP in cellular respiration. This metabolic process operates as a feedback loop. In this loop, downstream products control the speed of upstream reactions.
One enzyme involved in glycolysis is phosphofructokinase. It converts ADP to ATP. When there is too much ATP in the system, the ATP serves as an allosteric inhibitor. It binds to phosphofructokinase to slow down the conversion of ADP. In this way, ATP is preventing the unnecessary production of itself. There is no need to produce more ATP when there are already adequate amounts.
One example of an important drug that takes on the role of an allosteric inhibitor is the antibiotic penicillin. By helping the body kill off harmful bacteria, penicillin has saved millions of lives.
Harmful bacteria rely on the enzyme DD-transpeptidase to create strong, mesh-like cell walls. To counteract this process, penicillin binds to this enzyme. By acting as an inhibitor, penicillin prevents bacteria from building strong cell walls. With a weak wall, the surrounding fluids of the bacteria cell can then push itself in through osmosis. The cell will then burst and die.
Read Also: Movement Geography Definition
Quantitative Description Of Reversible Inhibition
Reversible inhibition can be described quantitatively in terms of the inhibitor’s binding to the enzyme and to the enzyme-substrate complex, and its effects on the kinetic constants of the enzyme. In the classic Michaelis-Menten scheme below, an enzyme binds to its substrate to form the enzymesubstrate complex ES. Upon catalysis, this complex breaks down to release product P and free enzyme. The inhibitor can bind to either E or ES with the dissociation constantsKi or Ki’, respectively.
|
Kinetic scheme for reversible enzyme inhibitors |