Transport Of Oxygen And Carbon Dioxide In The Blood
Transport of Oxygen:
- Erythrocytes carry oxygen in the blood.
- They are a biconcave shape, so there is a greater SA:V for diffusion.
- They have no nuclei, so space for oxygen is maximised.
- They have prosthetic haem groups, which have a high affinity for oxygen and combine reversibly to oxygen to absorb it/release it.
- When erythrocytes enter the lung capillaries, they are deoxygenated so there is a steep concentration gradient so the diffusion rate is high.
- When oxygen diffuses into erythrocytes, it forms a compound called oxyhaemoglobin . This also maintains a steep concentration gradient so diffusion rate into the cell remains high.
Oxygen Dissociation Curve:
- Shows how oxygen affinity changes with partial pressure.
- Should always form an s-shaped graph.
- Higher partial pressure, means a faster uptake of oxygen as it is more readily available, so there is a higher % oxygen saturation.
- In the lungs, there is high PPoO, and this steep concentration gradient causes a rapid loading of oxygen into hameoglobin.
- In the body, there is a drop in PPoO in the tissues, so this causes a rapid offloading of oxygen.
Key Terms
- Partial Pressure : The concentration of oxygen in a mixture of gases, e.g. the air.
- Bohr Effect: as partial pressure of carbon dioxide rises, haemoglobin gives away more oxygen.
Existing Theories Before Scheele
By the time he was a teenager, Scheele had learned the dominant theory of gases which in the 1770s was the phlogiston theory. Phlogiston, classified as “matter of fire”, was supposed to be released from any burning material, and when it was exhausted, combustion would stop. When Scheele discovered he called it “fire air” as it supported combustion. Scheele explained oxygen using phlogistical terms because he did not believe that his discovery disproved the phlogiston theory.
Before Scheele made his discovery of oxygen, he studied air. was thought to be an element that made up the environment in which took place but did not interfere with the reactions. Scheele’s investigation of air enabled him to conclude that air was a mixture of “fire air” and “foul air ” in other words, a mixture of two gases. Scheele performed numerous experiments in which he heated substances such as saltpetre , , heavy metal nitrates, and . In all of these experiments, he isolated the same gas: his “fire air,” which he believed combined with phlogiston in materials to be released during heat-releasing reactions.
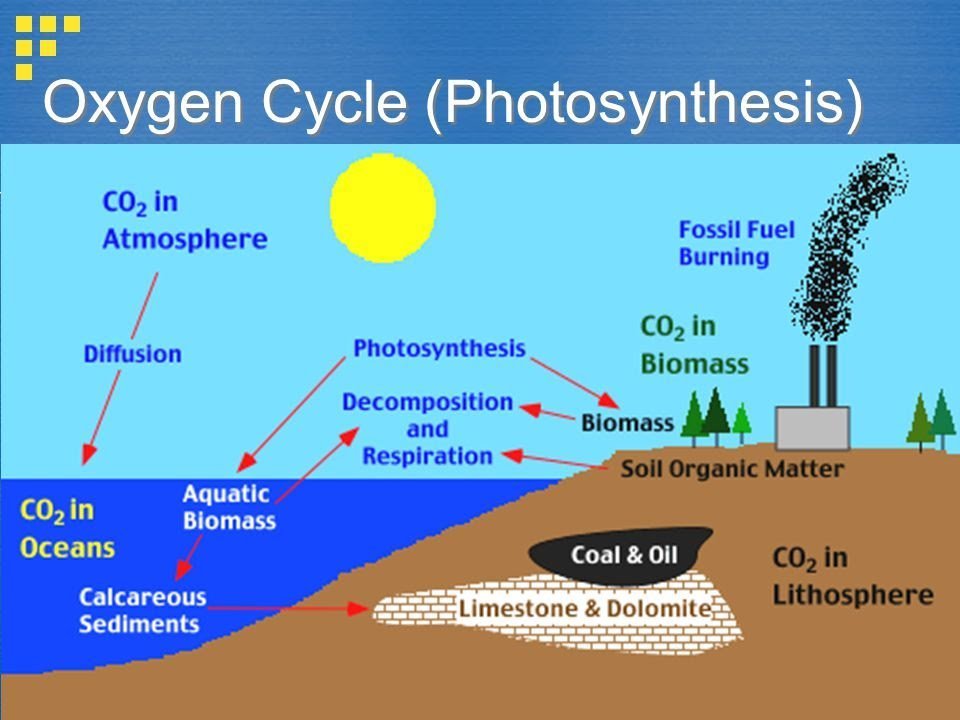
Stages Of The Oxygen Cycle
The steps involved in the oxygen cycle are:
Stage-1: All green plants during the process of photosynthesis, release oxygen back into the atmosphere as a by-product.
Stage-2: All aerobic organisms use free oxygen for respiration.
Stage-3: Animals exhale Carbon dioxide back into the atmosphere which is again used by the plants during photosynthesis. Now oxygen is balanced within the atmosphere.
Recommended Reading: Eoc Fsa Warm Ups Algebra 1 Answers
Tumor Cell Invasion Angiogenesis And Metastasis
After growth factor stimulation of RTKs, ROS can trigger activation of signaling pathways involved in cell migration and invasion such as members of the mitogen activated protein kinase family extracellular regulated kinase , c-jun NH-2 terminal kinase and p38 MAPK. ROS can also promote migration by augmenting phosphorylation of the focal adhesion kinase p130Cas and paxilin.
Both in vitro and in vivo, ROS have been shown to induce transcription factors and modulate signaling molecules involved in angiogenesis and metastasis .
Isotopes And Stellar Origin
Naturally occurring oxygen is composed of three stable isotopes, 16O, 17O, and 18O, with 16O being the most abundant .
Most 16O is synthesized at the end of the helium fusion process in massive stars but some is made in the neon burning process.17O is primarily made by the burning of hydrogen into helium during the CNO cycle, making it a common isotope in the hydrogen burning zones of stars. Most 18O is produced when 14N captures a 4He nucleus, making 18O common in the helium-rich zones of evolved, massive stars.
Fourteen radioisotopes have been characterized. The most stable are 15O with a half-life of 122.24 seconds and 14O with a half-life of 70.606 seconds. All of the remaining radioactive isotopes have half-lives that are less than 27 s and the majority of these have half-lives that are less than 83 milliseconds. The most common of the isotopes lighter than 16O is + decay to yield nitrogen, and the most common mode for the isotopes heavier than 18O is beta decay to yield fluorine.
Recommended Reading: How To Avoid Parallax Error In Physics
Oxides And Other Inorganic Compounds
Water is an oxide of hydrogen and the most familiar oxygen compound. Hydrogen atoms are covalently bonded to oxygen in a water molecule but also have an additional attraction to an adjacent oxygen atom in a separate molecule. These hydrogen bonds between water molecules hold them approximately 15% closer than what would be expected in a simple liquid with just van der Waals forces.
Due to its electronegativity, oxygen forms chemical bonds with almost all other elements to give corresponding oxides. The surface of most metals, such as aluminium and titanium, are oxidized in the presence of air and become coated with a thin film of oxide that passivates the metal and slows further corrosion. Many oxides of the transition metals are non-stoichiometric compounds, with slightly less metal than the chemical formula would show. For example, the mineral FeO is written as Fe }_}} , where x is usually around 0.05.
Oxygen is present in the atmosphere in trace quantities in the form of carbon dioxide . The Earth’s crustalrock is composed in large part of oxides of silicon , aluminium , iron _oxide” rel=”nofollow”> iron oxideFe2O3, in hematite and rust), and calcium carbonate . The rest of the Earth’s crust is also made of oxygen compounds, in particular various complex silicates . The Earth’s mantle, of much larger mass than the crust, is largely composed of silicates of magnesium and iron.
Water-soluble silicates in the form of Na4SiO
Transport Of Carbon Dioxide In The Blood
Carbon dioxide molecules are transported in the blood from body tissues to the lungs by one of three methods:
Several properties of carbon dioxide in the blood affect its transport. First, carbon dioxide is more soluble in blood than is oxygen. About 5 to 7 percent of all carbon dioxide is dissolved in the plasma. Second, carbon dioxide can bind to plasma proteins or can enter red blood cells and bind to hemoglobin. This form transports about 10 percent of the carbon dioxide. When carbon dioxide binds to hemoglobin, a molecule called carbaminohemoglobin is formed. Binding of carbon dioxide to hemoglobin is reversible. Therefore, when it reaches the lungs, the carbon dioxide can freely dissociate from the hemoglobin and be expelled from the body.
The benefit of the bicarbonate buffer system is that carbon dioxide is soaked up into the blood with little change to the pH of the system. This is important because it takes only a small change in the overall pH of the body for severe injury or death to result. The presence of this bicarbonate buffer system also allows for people to travel and live at high altitudes. When the partial pressure of oxygen and carbon dioxide change at high altitudes, the bicarbonate buffer system adjusts to regulate carbon dioxide while maintaining the correct pH in the body.
Also Check: Ccl4 Electron Geometry
Getting Oxygen Into The Body
The process by which living things exchange gases with their environment is known as respiration. Animal respiration takes place using specialized anatomical structures such as lungs or gills. Breathing, or ventilation, moves air into the lungs, which have membranes across which gases can diffuse — carbon dioxide from the bloodstream into lungs and oxygen in the other direction. In the bloodstream, oxygen binds to hemoglobin molecules, which transport the oxygen to the various cells of the body.
Chronic Inflammation And Cancer
Experimental and epidemiologic research over the past several years has indicated close associations among ROS, chronic inflammation, and cancer. ROS induces chronic inflammation by the induction of COX-2, inflammatory cytokines , IL-6), chemokines and pro-inflammatory transcription factors . These chemokines and chemokine receptors, in turn, promote invasion and metastasis of various tumor types.
Also Check: What Is Ap Biology In High School
Environmental Effects Of Oxygen
Highly concentrated sources of oxygen promote rapid combustion and therefore are fire and explosion hazards in the presence of fuels. The fire that killed the Apollo 1 crew on a test launchpad spread so rapidly because the pure oxygen atmosphere was at normal atmospheric pressure instead of the one third pressure that would be used during an actual launch.
Health Effects Of Oxygen
Oxygen is essential for all forms of life since it is a constituent of DNA and almost all other biologically important compounds. Is it even more drammatically essential, in that animals must have minute by minute supply of the gas in order to survive. Oxygen in the lungs is picked up by the iron atom at the center of hemoglobin in the blood and thereby transported to where it is needed.
Every human being needs oxygen to breathe, but as in so many cases too much is not good. If one is exposed to large amounts of oxygen for a long time, lung damage can occur. Breathing 50-100% oxygen at normal pressure over a prolonged period causes lung damage. Those people who work with frequent or potentially high exposures to pure oxygen, should take lung function tests before beginning employment and after that. Oxygen is usually stored under very low temperatures and therefore one should wear special clothes to prevent the freezing of body tissues.
Also Check: What Does Abiotic Mean In Biology
Polymeric Vs Monomeric Molecular Structures
Oxides of most metals adopt polymeric structures with M-O-M crosslinks. Because these crosslinks are strong, the solids tend to be insoluble in solvents, though they are attacked by acids and bases. The formulas are often deceptively simple. Many are nonstoichiometric compounds. In these oxides, the coordination number of the oxide ligand is 2 for most electronegative elements, and 36 for most metals.
Silicon Dioxide: Silicon dioxide is one of the most common oxides on the surface of earth. Like most oxides, it adopts a polymeric structure.
Although most metal oxides are polymeric, some oxides are monomeric molecules. The most famous molecular oxides are carbon dioxide and carbon monoxide. Phosphorus pentoxide is a more complex molecular oxide with a deceptive name, the formula being P4O10. Some polymeric oxides depolymerize to give molecules when heated. Tetroxides are rare, and there are only five known examples: ruthenium tetroxide, osmium tetroxide, hassium tetroxide, iridium tetroxide, and xenon tetroxide. Many oxyanions are known, such as polyphosphates and polyoxometalates. Oxycations are rarer, an example being nitrosonium . Of course many compounds are known with both oxides and other groups. For the transition metals, many oxo-complexes are known, as well as oxyhalides.
Life Support And Recreational Use
An application of O2 as a low-pressure breathing gas is in modern space suits, which surround their occupant’s body with the breathing gas. These devices use nearly pure oxygen at about one-third normal pressure, resulting in a normal blood partial pressure of O2. This trade-off of higher oxygen concentration for lower pressure is needed to maintain suit flexibility.
Scuba and surface-suppliedunderwater divers and submariners also rely on artificially delivered O2. Submarines, submersibles and atmospheric diving suits usually operate at normal atmospheric pressure. Breathing air is scrubbed of carbon dioxide by chemical extraction and oxygen is replaced to maintain a constant partial pressure. Ambient pressure divers breathe air or gas mixtures with an oxygen fraction suited to the operating depth. Pure or nearly pure O2 use in diving at pressures higher than atmospheric is usually limited to rebreathers, or at relatively shallow depths , or medical treatment in recompression chambers at pressures up to 2.8 bar, where acute oxygen toxicity can be managed without the risk of drowning. Deeper diving requires significant dilution of O2 with other gases, such as nitrogen or helium, to prevent oxygen toxicity.
Other recreational uses that do not involve breathing include pyrotechnic applications, such as George Goble‘s five-second ignition of barbecue grills.
Read Also: Paris Jackson Mom And Dad
Geologic History Of Oxygen
3.85 to 2.45 billion years ago, there was no free oxygen yet in the Earths atmosphere and most oceanic parts were anoxic. Free oxygen began to exist in the atmosphere when photosynthetic organisms evolved. This is believed to have occurred in about 3.5 billion years ago. Through photosynthesis, they utilized carbon dioxide, water, and photons to yield sugars. The oxygen produced from photosynthesis as well was discarded as a waste product. In 2.45 to 1.85 million years ago, oxygen level started to significantly rise. Much of the free oxygen produced by organisms was absorbed in oceans and seabed rock. The biologically-induced oxygen buildup has been referred to as the Great Oxygenation Event. It is surmised to have occurred during the Siderian period of the Paleoproterozoic Era. The significant buildup of free oxygen caused the extinction of many obligate anaerobes.
Free oxygen began to outgas from the oceans 1.85 to 0.85 billion years ago. The land surfaces absorbed much of it. From then on to the present, free oxygen eventually accumulated in the atmosphere, especially when oxygen reservoirs were filled. The evolution of organisms that could metabolize oxygen curbed the increment of available free oxygen.
Gas Pressure And Respiration
The respiratory process can be better understood by examining the properties of gases. Gases move freely, but gas particles are constantly hitting the walls of their vessel, thereby producing gas pressure.
Air is a mixture of gases, primarily nitrogen , oxygen , water vapor , and carbon dioxide . Each gas component of that mixture exerts a pressure. The pressure for an individual gas in the mixture is the partial pressure of that gas. Approximately 21 percent of atmospheric gas is oxygen. Carbon dioxide, however, is found in relatively small amounts, 0.04 percent. The partial pressure for oxygen is much greater than that of carbon dioxide. The partial pressure of any gas can be calculated by:
Patm, the atmospheric pressure, is the sum of all of the partial pressures of the atmospheric gases added together,
The pressure of the atmosphere at sea level is 760 mm Hg. Therefore, the partial pressure of oxygen is:
and for carbon dioxide:
At high altitudes, Patm decreases but concentration does not change the partial pressure decrease is due to the reduction in Patm.
When the air mixture reaches the lung, it has been humidified. The pressure of the water vapor in the lung does not change the pressure of the air, but it must be included in the partial pressure equation. For this calculation, the water pressure is subtracted from the atmospheric pressure:
and the partial pressure of oxygen is:
Also Check: Holt Mcdougal Geometry Workbook Answers
The Theory Of Phlogiston
Chemical Treatise on Air and Fire
Scheele achieved astonishingly prolific and important results without the expensive laboratory equipment to which his Parisian contemporary Antoine Lavoisier was accustomed. Through the studies of Lavoisier, Priestley, Scheele, and others, was made a standardized field with consistent procedures. Although Scheele was unable to grasp the significance of his discovery of the substance that Lavoisier later named oxygen, his work was essential for the abandonment of the long-held theory of phlogiston.
Scheele’s study of the gas not yet named oxygen was prompted by a complaint by , a professor at who would eventually become Scheele’s friend. Bergman informed Scheele that the saltpeter he had purchased from Scheele’s employer, after long heating, produced red vapors when it came into contact with acetic acid. Scheele’s quick explanation was that the saltpeter had absorbed phlogiston with the heat and gave off a new phlogisticated gas as an active principle when combined with an acid .
When other chemists later showed water is produced when burning hydrogen and that rusting of metals added weight to them and that passing water over hot iron gave hydrogen, Scheele modified his theory to suggest that oxygen was the salt , and that when added to iron, water was reproduced, which added weight to the iron as rust.
About This Research Topic
Reactive oxygen species are defined as relatively short-lived molecules that contain oxygen atoms, showing half-lives in the range of nanoseconds to hours. They are produced as molecules, ions, and radicals in many chemical and biological processes, such as hydrogen peroxide …
Keywords:reactive oxygen species, stress-oxidative activity, hybrid redox-active nanomaterials, biomedicine, photoactivity, advanced oxidation processes
Important Note: All contributions to this Research Topic must be within the scope of the section and journal to which they are submitted, as defined in their mission statements. Frontiers reserves the right to guide an out-of-scope manuscript to a more suitable section or journal at any stage of peer review.
You May Like: Find The Length Indicated Geometry
O2 Adsorption And Reduction
Molecular oxygen is important in TiO2-based photocatalysis because it can act as an electron scavenger when it is adsorbed on the surface. In principle, this suppresses electronhole recombination in the semiconductor and increases the yield of the photocatalytic reactions. However, the efficiency of oxygen as an electron scavenger is rather limited. One of the points that have been studied to explain this behavior is the nature of the reduced, adsorbed species, namely whether it is superoxide or peroxide . Electron paramagnetic resonance experiments indicate that the preferred form is the superoxide.
This question has been studied with periodic PBE0 calculations on a system formed by an anatase surface slab with two excess electrons, denoted as * below, and an oxygen molecule.14 The studied process is as follows:
The results show that the peroxide configuration where the two electrons localize on the oxygen molecule is approximately 0.8 eV more stable than the superoxide configuration with one electron in the surface and one on the adsorbate. However, the second electron transfer step, that is, the conversion of the superoxide to a peroxide, is nonadiabatic and involves a barrier of approximately 0.3 eV. This suggests that the superoxide is kinetically stabilized by the barrier, which in turn limits the efficiency of oxygen as an electron scavenger.
S.T. Petsch, in, 2014