What Are Amorphous Solids
The substances that appear like solids but do not have well developed perfectly ordered crystalline structures are called amorphous solids. Tar, glass, plastic, rubber, butter etc. are classified as amorphous solids. Amorphous solids do not have an ordered internal structure and do not melt at a definite, sharp melting point. With an increase in temperature, it gradually softens, becomes less viscous and melts over a range of temperatures.
Amorphous solids are not solids in the real sense. Truly they are supercooled liquids. The regular arrangement of constituent particles is present only up to a short distance in an amorphous solid. The regular and periodically repeating pattern is not observed in amorphous solid. The structure of amorphous solids is similar to the structure of liquids. Amorphous solids behave like fluids and very slowly float under gravity. Due to the
property of liquids, fluidity, the lower sides of glass panes of windows, photo frames, cupboards and showcases become slightly thicker and upper ends become thinner.
Amorphous solids are also called pseudo solids or supercooled liquids. Like crystalline solids, the values of physical properties of amorphous solids do not change with the change of direction. The properties that remain the same in all directions is called isotropy.
Example: Rubber, plastic, gels, glass, polymers, gel, fused silica, pitch tar, thin film lubricants, wax.
Determining The Difference Between Crystalline & Amorphous Solids
The fundamental difference between crystalline and amorphous compounds is the arrangement of their constituent atoms. A crystalline solid has a long range of ordered molecules and a sharp melting point. In contrast, an amorphous compound has a short range of ordered molecules and an irregular arrangement of its atoms. This makes amorphous compounds highly rigid and have irregular surfaces.
Amorphous solids are non-crystalline substances that do not possess a characteristic geometrical arrangement. They also do not have a fixed melting point. Despite their name, amorphous materials do display an orderly arrangement of atoms that may extend up to a few Angstrom units. Such structures are called crystallites. Amorphous solids lack distinct edges and are therefore non-crystalline.
Amorphous solids lack a long-range arrangement of atoms. In addition, they do not have any characteristic geometry. They are identical in all their properties along all their axes. Because of this, they are impossible to identify by their structure as a crystalline one. The distinction between amorphous and crystalline solids is very useful when describing the properties of different materials.
Amorphous solids are characterized by irregular breakage. They do not have definite melting points. They are homogeneous, asymmetrical, and have no sharp edges. Amorphous materials do not have a defined shape. Unlike crystalline solids, amorphous solids do not show long-range order. They are anisotropic.
Question: 9is The Wood Amorphous Or Crystalline
Answer:
The given solid crystals are made of various substances like stone, wood, paper and cloth. Such solids are composed of atoms arranged in a certain order. The transition to a liquid, called melting, is as sharp and transparent as the solid crystals are heated. Amorphous solids are made of rubber, glass, and sulfur.
Read Also: Prentice Hall Foundations Geometry 12 1
Crystalline Solids And Amorphous Solids
Crystalline solids and amorphous solids are the two types of solid formed by atoms, ions, or molecules. Molecular, ionic, covalent, and metallic crystalline solid are the main types of crystals formed by their constituents with a definite structure. An amorphous solid is a material that does not possess a definite structure and sharp melting point.
In chemistry, a solid molecule is characterized by its definite shape, strength, density, and rigidity rather than liquid and gases. Solids are rigid due to the absence of translatory motion on the structural unit. These units are fixed to their mean position with strong forces of attraction.
What Is The Reason For Isotropy In Amorphous Solids

In my book, it’s been mentioned that crystalline solids are anisotropic whereas amorphous solids are isotropic in nature. The reason for these has been explained as:
Crystalline solids are anisotropic in nature, that is, some of their physical properties like electrical resistance or refractive index show different values when measured along different directions in the same crystals. This arises from different arrangement of particles in different directions. Since the arrangement of particles is different along different directions, the value of the same physical property is found to be different along each direction.
Amorphous solids on the other hand are isotropic in nature. It is because there is no long range order in them and arrangement is irregular along all directions. Therefore, value of any physical property would be same along any direction.
My question is: similar arrangement of particles in different directions in crystalline solids gives rise to different physical properties. Then, how can disarrangement in amorphous solids in all directions give rise to same physical properties?
In other words, why do crystalline solids have properties different in different directions, and the amorphous solids have these properties same in all directions?
Read Also: How To Do Unit Conversions In Chemistry
Solved Examples For You
Question: Assertion- Initially the term pseudo solid was given for solids, which were easily distorted by bending and compression forces. They even tend to flow slowly under its own mass and lose shape.
Reason-
Solution: Option B. Both Assertion and Reason are Correct but the Reason is not the correct explanation for the Assertion. Initially, the term pseudo solid was given for amorphous solids, which were easily distorted by bending and compression forces. They even tend to flow slowly under its own mass and lose shape.
The intermolecular forces present in such materials are stronger than those present in the liquids but weaker than those present in solids. They are not solids in the real sense. The regular arrangement of constituent particles is present only up to short distance in these solids. These characteristics are shown by pseudo solids as in pitch, glass and thus, the name pseudo solid was replaced by supercooled liquids.
Is Coal Crystalline Or Amorphous
Graphite is one of three types of carbon which is crystalline, or crystal-forming. In compounds like coal and charcoal, carbon also occurs as an amorphous, or shapeless, form. Allotropes are called varying variants of the same substance. Some allotropes of crystalline carbon are, aside from graphite, diamond, and fullerenes.
Also Check: What Does Site Mean In Geography
Preparation By Ion Implantation
One way to produce a material without an ordered structure is to take a crystalline material and remove its internal order by damaging it. A practical, controllable way to do this is by firing ions into the material at high speed, so that collisions inside the material knock all atoms from their original positions. This technique is known as ion implantation. It produces amorphous solids only if the material is too cold for atoms to diffuse back to their original positions as the process continues.
Magnetic Properties Of Amorphous Solids
Amorphous materials showing ferromagnetism and ferrimagnetism include alloys of a transition metal and a metalloid, and of a transition metal and a rare earth. In these materials, a long-range order exists in the ensemble of magnetic dipole moments although the arrangement of constituting atoms is disordered. Thus far, many amorphous alloys have been prepared and their magnetic properties and structures have been examined. According to the previous studies, the ferromagnetic amorphous alloys possess well-defined Curie temperatures, and the second-order phase transition between ferromagnetic and paramagnetic phased is observed, where the critical exponents can be defined . The ferromagnetic states and the magnetic transition are intrinsically the same as those of ferromagnetic crystalline materials. The ferromagnetic amorphous alloys can be used as a magnetic core and a magnetic head because of their excellent soft magnetic properties.
G. Herzer, in, 2001
You May Like: Common Core Algebra 2 Unit 3 Linear Functions Answer Key
Summary Amorphous Vs Crystalline Solid
Solids are mainly in three types as amorphous, semi-crystalline and crystalline solids. The key difference between amorphous and crystalline solid is that the crystalline solids have an ordered long-range arrangement of atoms or molecules within the structure, whereas the amorphous solids lack ordered long-range arrangement.
Reference:
1. Douglas, Ronald Walter, et al. Amorphous Solid. Encyclopædia Britannica, Encyclopædia Britannica, Inc., 22 Apr. 2016. Available here 2. Libretexts. 12.1: Crystalline and Amorphous Solids. Chemistry LibreTexts, National Science Foundation, 26 Nov. 2018. Available here
Image Courtesy:
1.Crystalline or amorphousBy Sbyrnes321 via Commons Wikimedia 2.148812By OpenClipart-Vectors via pixabay
Different Types Of Solids
Solids are divided into two categories depending on their essential structures. They can be crystalline solids or noncrystalline amorphous materials, depending on whether their structure is regular or disordered.
Almost every material may be rendered amorphous by rapidly cooling it from its liquid state, however certain materials are inherently amorphous because their constituent atoms or molecules cannot fit together in a regular manner. Other materials are amorphous because they have faults or impurities that prevent a stable lattice from forming.
The molecules or atoms in crystalline solids are organized in a repeating pattern called a lattice structure. A unit cell is the smallest repeating unit in that lattice arrangement. Solids of this sort are the most prevalent. They frequently split into flat faces and geometric forms when they crack.
Long-range order does not exist in amorphous solids. This implies that the pattern of atoms or molecules in one region of the solid will vary hugely from the pattern in another. Most amorphous solids, on the other hand, exhibit short-range order: At the molecular level, an image of a very small section of a solid may appear to be organized.
Read Also: Ratios And Proportions Algebra 1
Structure Of Crystalline Solids
Crystalline solids have well-defined edges and faces with definite melting points. The study of the geometrical structure in the crystal lattice is called crystallography.
The structure of the sodium chloride crystal is given below the picture,
Bragg diffraction experiment in physics or chemistry is very useful for the determination and analysis of crystal structure. It is developed from the very simple relation between wavelengths of the x-ray radiation and spacing between the two lattice planes.
No Sharp Melting Point
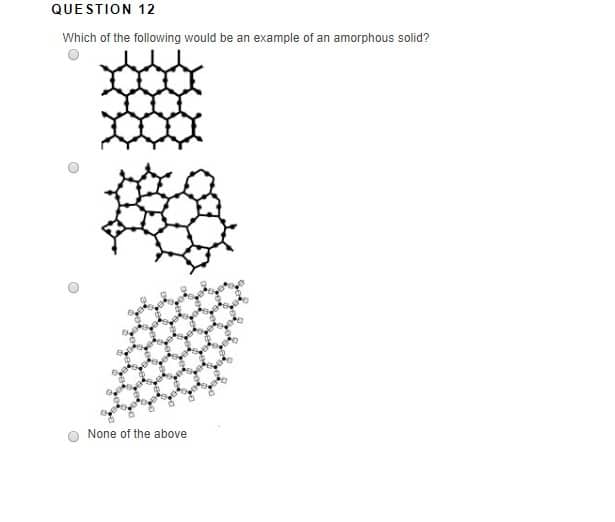
An amorphous solid does not have a sharp melting point but melts over a range of temperatures. For example, glass on heating first softens and then melts over a temperature range. Glass, therefore, can be moulded or blown into various shapes. Amorphous solid does not possess the characteristic heat of fusion.
Don’t Miss: Abstract Algebra Judson Solutions Manual
Preparation And Alloy Systems
Amorphous materials can be principally synthesized by a variety of techniques such as rapid solidification from the liquid state, mechanical alloying, plasma processing, and vapor deposition. For soft magnetic applications the material is mostly produced by rapid solidification from the melt as thin ribbons usually about 2030 m thick and about 1100 mm wide.
Nanocrystalline soft magnetic alloys, actually, are also cast as an amorphous ribbon which is subsequently annealed above its crystallization temperature to produce the nanocrystalline state. Actually, controlled crystallization from the amorphous state seems to be the only method presently available to synthesize nanocrystalline alloys with attractive soft magnetic properties.
The range of compositions which can be prepared in the glassy state by rapid solidification from the melt is wide. Typical compositions are given by the formula T7090X1030 . Here, T stands for a practically arbitrary combination of transition metals which, for magnetic applications, are of course given by Fe, Co, and Ni. The letter X refers to metalloid atoms like Si and B and/or refractory metals like Nb, Mo, Zr, Hf, etc. These nonmagnetic additions are necessary for glass formation and in order to stabilize the amorphous structure.
The final alloy design is largely determined by the desired magnetic properties, good glass forming ability, thermal stability, and, for the nanocrystalline alloys , a well-defined crystallization behavior.
Characterization Of Amorphous Solids
The strategy for characterizing amorphous solids differs from that for crystalline solids. Molecular level structural elucidation, as is feasible for crystalline solis by diffraction and spectroscopic methods, is less applicable to amorphous solids, and greater emphasis is placed on structural mobility and changes. It is customary to characterize an amorphous material both below and above the glass transition temperature . The physical characterization of amorphous solids utilizes a wide range of techniques and offers several types of information:
Don’t Miss: Which Essential Element Of Geography Focuses On Common Cultures
Understand What Amorphous Means In Science
- Ph.D., Biomedical Sciences, University of Tennessee at Knoxville
- B.A., Physics and Mathematics, Hastings College
In physics and chemistry, amorphous is a term used to describe a solid which does not exhibit crystalline structure. While there may be local ordering of the atoms or molecules in an amorphous solid, no long-term ordering is present. In older texts, the words “glass” and “glassy” were synonymous with amorphous. However, now glass is considered to be one type of amorphous solid.
Is Wood Amorphous Or Crystalline
Crystalline solids are made of stone, wood, paper and cloth. Such solids consist of atoms arranged in a particular fashion. The transition to liquid, called melting, is sharp and transparent as crystalline solids are heated. Amorphous solids are made of rubber, glass, and sulphur.
Have a pressing doubt regarding the subject? Try the live chat for any questions anytime and our consultants will get back to you.
Put your understanding of this concept to test by answering a few MCQs. Click Start Quiz to begin!
Select the correct answer and click on the Finish buttonCheck your score and answers at the end of the quiz
Recommended Reading: What Is Cross Cultural Psychology
Can Be Converted Into Crystalline Solids
When you heat amorphous solids and then leave them to cool down slowly through the process of annealing, they take on the shape of crystalline solids at a specific temperature. Therefore, some ancient glass can be found that looks milky because of the crystallization that has taken place inside it over the years.
Amorphous Solids Are Isotropic
Amorphous solids are isotropic. That is, they exhibit uniform properties in all directions. The thermal and electrical conductivities, coefficient of thermal expansion and refractive index of an amorphous solid have the same value in whatever direction the properties are measured.
Any given crystalline solid can be made amorphous by the very rapid cooling of its melt or by freezing its vapour. This does not allow the particles to arrange themselves in a crystalline pattern. When quartz the crystalline form of SiO2 is melted and then rapidly cooled, an amorphous solid known as quartz glass or silica glass results. This material has the same composition SiO2 but lacks the molecular level orderliness of quartz. Amorphous form of metal alloys are obtained when thin films of melted metal are rapidly cooled. The resulting metallic glasses are strong, flexible and much more resistant to corrosion than the crystalline alloys of the same composition.
Read Also: What Does Yer Mean In Math
What Is Amorphous And Amorphous Examples
Amorphous Meaning: The solid is amorphous when the particles used do not have a three-dimensional action mechanism. Amorphous solids, with the exception of the application of a three-dimensional spectrum of glass-like material, have an unusual game of particles, show short-range application with a small number of atoms, and have completely different physical properties for their flexible comparisons. The solid appearance of amorphous molecules is like a liquid because they do not have a fixed structure, a formal system of atoms or ions in a three-dimensional structure.
These solids do not have a sharp point of dissolution and solids in liquid conversion occur above temperatures. The physiological features exhibited by amorphous intensity are usually isotropic as the structures do not depend on the measurement index and show the same degree in different directions. In this article, we will learn about amorphous solid, the difference between crystal and amorphous solid, strong chemical properties, characteristics of amorphous solid, and what is an amorphous form.
Also read –
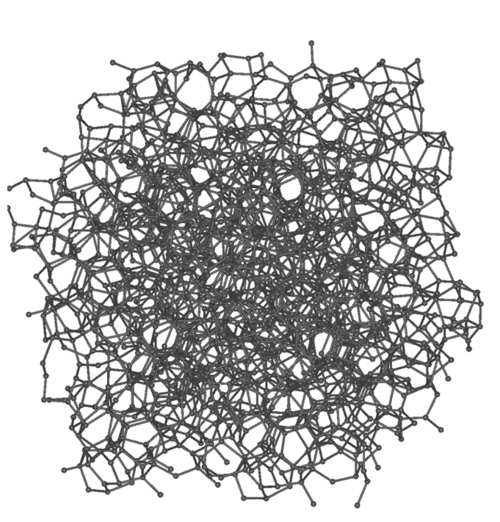
Crystalline And Amorphous Solids
Learning Objectives
- To understand the difference between a crystalline and an amorphous solid
Crystalline solids have regular ordered arrays of components held together by uniform intermolecular forces, whereas the components of amorphous solids are not arranged in regular arrays. The learning objective of this module is to know the characteristic properties of crystalline and amorphous solids.
Also Check: What Is The Definition Of Macromolecules In Biology
Summary Of Crystalline Verses Amorphous
- Crystalline solids have a regular three-dimension pattern of ions, atoms or molecules whereas amorphous solids have a random arrangement of these components
- Crystalline solids have an exact melting point whereas amorphous solids melt over a range of temperature
- Amorphous solids yield fragments with irregular patterns when cleaved whereas crystalline solids depict a definite shape
- Crystalline called anisotropic because of different physical properties in all directions whereas amorphous are called isotropic
- Examples of amorphous include glass and that of crystalline include diamond.
Definition Of Amorphous Solids
The definition of amorphous must be easily understood, accessible and tangible for criminal purposes. Amorphous solids are like liquids in that they have no ordered structure, the order of atoms or ions in a three-dimensional structure. These solids have no sharp melting point and solids in liquid conversion occur at a certain temperature. Physical features characterized by amorphous hardness are generally isotropic as the structures do not depend on the direction of scale and show the same size in different ways.
|
Related Topics link |
You May Like: What Does All Real Numbers Mean In Algebra
Preparation Of Metallic Glass
Some amorphous metallicalloys can be prepared under special processing conditions, such as rapid solidification, thin-film deposition, or ion implantation. The term “metallic glass” refers only to amorphous metallic alloys that have been rapidly solidified. Materials produced by ion implantation or thin-film deposition are technically not called metallic glasses.
Even with special equipment, such rapid cooling is required that, for most metals, only a thin wire or ribbon can be made amorphous. This is adequate for many magnetic applications, but thicker sections are required for most structural applications such as scalpel blades, golf clubs, and cases for consumer electronics.
Recent efforts have made it feasible to increase the maximum thickness of glassy castings, by finding alloys that have greater kinetic barriers to crystallization. Such alloy systems tend to have the following interrelated properties:
- Many different solid phases are present in the equilibrium solid, so that any potential crystal will find that most of the nearby atoms are of the wrong type to join in crystallization.
- The composition is near a deep eutectic, so that low melting temperatures can be achieved without sacrificing the slow diffusion and high liquid viscosity found in alloys with high-melting pure components.
- Atoms with a wide variety of sizes are present, so that “wrong-sized” atoms interfere with the crystallization process by binding to atom clusters as they form.