Acids And Bases In Organic Chemistry
Acids and bases are crucial when it comes to organic chemistry.
Not those crazy ice charts and pKa calculations. At the organic chemistry level you will be asked to differentiate and rank acids and bases by looking at their molecular structure and comparing their reactions.
This shows up early in Orgo 1 when you learn the material, then again when ranking the reactivity of bases in elimination reactions, and again in Orgo 2 when studying stability of complex molecules.
As an orgo tutor I am often frustrated by the manner in which this material is taught. My students come to me all confused with data they memorized but don’t understand. And so I created what I hope is a complete resource to help you really GET acids and bases.
Be sure to watch each video to learn the information with a concept/trend focus. If you’d like to see this subject with more focus on the math, see my MCAT Acid/Base Tutorial Series. Then download my FREE Acid Base Cheat Sheet to take your studies on the go. And when you feel ready, see how you do on my Acid Base Practice Quiz.
Don’t forget to grab the Strong Acid/Base Mini Cheat Sheet.
Examples Of Brnsted Acid
| Acid-Base Reaction |
|---|
| Kw = = 10-14 |
The range of strong acids that can be distinguished is limited. Thus acids that are stronger than the hydronium cation, H3O, and weak acids having conjugate bases stronger than hydroxide anion, OH, cannot be measured directly in water solution. If a strong acid has a Ka = 100, a 0.1M solution in water will ionize completely, giving a hydronium concentration of 100 and a concentration of 10-4. A similar solution of a 103 times stronger acid would be undetectably different due to this leveling effect of the solvent. The term leveling effect refers to the influence an amphoteric solvent such as water has on measurements of acidity and basicity. The strength of a strong acid is leveled by the basicity of the solvent, and the basicity of strong bases is likewise limited by solvent acidity.
Since many organic reactions either take place in aqueous environments , or are quenched or worked-up in water, it is important to consider how a conjugate acid-base equilibrium mixture changes with pH. A simple relationship known as the Henderson-Hasselbalch equation provides this information.
| Acid-Base Reaction | |
|---|---|
| Ka = 5.5*10-10 | pKa = 9.25 |
| Compound |
|---|
| -10.0 |
Example Question #: Help With Acid Base Reactions
Rank these weak acids by decreasing .
The governing principle regarding the prediction of values is to assess the stability of the product formed by the release of a proton. The release of the alkyne hydrogen in compound III results in a carbanion, a highly unstable species, so it is expected that this compound is the least acidic and has the highest . Intuition serves well in this instance and we see that hydrogens bound to a triple bond have a value of around 25. The relative stabilities of the remaining compounds may be assessed in the same manner. Compound IV is the second weakest acid because the three methyl groups donate electron density such that if the oxygen is deprotonated, the resulting negative charge is destabilized. Methanol and water have a unique, non-intuitive relationship regarding their relative acidities. One would assume that water should be a stronger acid than other acids bound to alkyl groups . This is the case for all alcohols except methanol, in which the delocalization of charge allowed by the increased molecular size outweighs the destabilization caused by electron donation. Thus methanol is a slightly stronger acid than water. This is evidenced in their values: 15.7 for water and 15.5 for methanol. The correct ordering of the given compounds is: III, IV, II, I.
Also Check: What Does Political Geography Mean
Order Of Acidity In Aromatic Molecules With Various Functional Groups
What factors need to be considered in order to rank the acidity of aromatic molecules with various substituents?
Given benzene with 1 $\ce$ group. How does adding the following functional groups affect acidity, and what are the rules to determine this?
- an additional $\ce$ group
- $\ce$
- $2\times\ce$ groups
- $3\times\ce$ groups
Based on what I know about acids, the following increase acidity:
- charge – the less electrons, the more acidic
- electronegativy increases.
- compounds with more resonance – more stability.
- more electron withdrawing groups.
- lower orbitals – the more s character , the closer the electrons are to the nucleus, and the lower the energy, which means more stability.
The following decreases acidity
- steric hindrance – where 3D structure of a molecule make solvent less accessible, and thus more weakly stabilised.
- larger molecules tend to require a larger number of solvent molecules to solvate them.
Ology For The Study Of Ntot
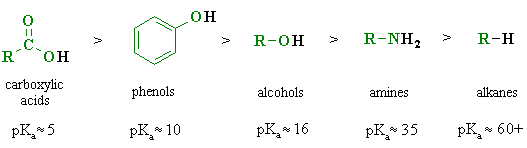
The seasonal variations in Ntot were explored based on the comparison ofthe seasonal medians. For simplicity, seasons were assigned using the commonDecemberFebruary , MarchMay , JuneAugust andSeptemberNovember division at all sites, even for the stations whereother time divisions would be more appropriate. This is the case, forinstance, at CHC, where the weather is affected by two main seasons with tropical characteristics . Such specificities should be kept in mind wheninterpreting the results.
Recommended Reading: Abiotic Features Definition
Acidic And Acidity Of Organic Compounds And Organic Chemistry
In this lesson, we learn about acidity of differentorganic compounds and which organic compounds show acidic characteristics. Acidity of organic compounds depends on many different factors. Some organic compounds are very acidic compared to some organic compounds. Also there are basic organic compounds such as amine compounds.
At the end of this tutorial, you should have the ability to decide which organic compound is more acidic from given compounds and what are the reasons for that. We study all acidic organic compounds in this lesson.
General Assessment Of Acidities
When considering alcohols as organic reagents, pKas are often used because they reflect reactivity in aqueous solution. In general, alcohols in aqueous solution are slightly less acidic than water. However, the differences among the pKas of the alcohols are not large. This is not surprising because all alcohols are oxy-acids , and the differences in acidities are due to the effect of substituents in the 1-position removed from the acidic site. Moreover, the more highly substituted alcohols vary only in the structure two positions removed from the acidic site. The marginal effects of additional substituents at the carbon tow positions removed from the acidic site are even evident in the gas-phase enthalpies of reaction for the reaction
The pKas and gas-phase enthalpies of reaction for various alcohols, ROH, with various substituents are shown in Table 1 below.
Table 1: pKas and gas-phase enthalpies of reaction
| R | \ |
|---|---|
| H | |
| 1462 ± 10 |
Read Also: Example Of Span Linear Algebra
Termination And Transfer On Reactive Molecules
The very strong basicity of carbanionic active centres is responsible for their termination with electrophilic sites. All proton donors react instantaneously to give the polymeric hydrocarbon .
Organolithium derivatives are unstable in ethers due to the too high acidity of protons in the position being too high with respect to oxygen. A mechanism similar to that proposed by Rembaum et al.19 for decomposition of ethyllithium in THF is generally accepted .
Scheme 5.
Polymerizations performed in liquid ammonia and initiated by either Na blue solutions or sodium amide, lead to transfer to solvent .22 It has been reported21 that for polymerizations performed in toluene alone, transfer to solvent can also take place , the benzyllithium formed reinitiating polymerization.
Such a reaction has been used to synthesize low molecular weight polybutadiene. Polymerizations initiated by lithium or sodium derivatives, in the presence of toluene and THF, lead to phenyl-terminated polybutadiene with Mn from 700 to 104 depending on the conditions of polymerization.23
Living anionic centres react readily with atmospheric impurities. With oxygen, a complex reaction occurs24 which has been comprehensively studied by Brossas and co-workers.25 A coupling reaction, revealed by GPC,26,27 occurs simultaneously with peroxidation, the mechanism of which was identified using low molecular weight models . The proportions of various species formed are closely related to the oxidation conditions.25
Factor #2 The Role Of The Atom
This point causes a lot of confusion due to the presence of two seemingly conflicting trends.
Heres the first point: acidity increases as we go across a row in the periodic table. This makes sense, right? It makes sense that HF is more electronegative than H2O, NH3, and CH4 due to the greater electronegativity of fluorine versus oxygen, nitrogen, and carbon. A fluorine bearing a negative charge is a happy fluorine.
But heres the seemingly strange thing. HF itself is not a strong acid, at least not in the sense that it ionizes completely in water. HF is a weaker acid than HCl, HBr, and HI. Whats going on here?
You could make two arguments for why this is. The first reason has to do with the shorter H-F bond as compared to the larger hydrogen halides. The second has to do with the stability of the conjugate base. The fluoride anion, F is a tiny and vicious little beast, with the smallest ionic radius of any other ion bearing a single negative charge. Its charge is therefore spread over a smaller volume than those of the larger halides, which is energetically unfavorable: for one thing, F begs for solvation, which will lead to a lower entropy term in the G.
Note that this trend also holds for H2O and H2S, with H2S being about 10 million times more acidic.
Read Also: Who Is Generally Recognized As The Founder Of American Psychology
The Attempt At A Solution
get_physical said:Br is lower on the periodic table, hence more acidic.
Borek said:In HX compounds I would agree, but here what matters is the electronegativity – whatever is more electronegative will pull the electrons stronger, lowering electron density on the -COOH group and making the acid stronger.
not the rule where going down is more acidic?
- 0
- 28,822
- 3,340
Borek said:I have a feeling you are mixing up several separate trends . As far as I can tell “haloacid” is a name used for halogenated organic acid, so how can you have haloacid that is “without COOH group”?Strength of HX acids is HI > HBr > HCl > HF – that’s where the acidity grows down the period. But it doesn’t mean haloacids will behave the same way, as their acidity depends on completely different effects.
get_physical said:So for Br-CH2-COOH and F-CH2-COOH, which one is more acidic?
We said that F-CH2-COOH is more acidic because F is more electronegative, but at the same time, “Strength of HX acids is HI > HBr > HCl > HF ”
- 85
- 0
according to the “Strength of HX acids is HI > HBr > HCl > HF “???
HF is not. It is a hydrogen halide.
- 398
- 22
You are confusing the stabilization due to the inductive effect in the haloacid case versus the concept of anion charge density in the case of hydrogen halides.
- 28,822
- 3,340
- 398
- 22
Which Compound Is Most Acidic Benzenealkene
Both solutions are do not show specific acidic characteristics. Benzene and alkene do not react with sodium, aqueous sodium hydroxide, aqueous sodium carbonate, aqueous sodium bicarbonate. Usually acidic organic compounds react with one or more of sodium, aqueous sodium hydroxide, aqueous sodium carbonate, aqueous sodium bicarbonate.
Recommended Reading: Elton John Children Biological
Acid Strength And Pka
Now that we know how to quantify the strength of an acid or base, our next job is to gain an understanding of the fundamental reasons behind why one compound is more acidic or more basic than another. This is a big step: we are, for the first time, taking our knowledge of organic structure and applying it to a question of organic reactivity. Many of the ideas that well see for the first here will continue to apply throughout the book as we tackle many other organic reaction types.
Impact On The Estimation Of Dcy
Based on these last observations, and even if long interruptions were the main reason for decreased data availabilityin the datasets , the coverage criteria were raised to 75% forthe study of Dcy, and the main analysis was limited to the annual scale.The seasonal change in the diel cycle was only briefly investigated at a fewsites with particularly high coverage to give further insight into thefindings obtained at the annual scale. All the results presented in Sect. 6should nonetheless be considered with caution, as the length of the selecteddatasets in any case remains limited for such an application.
Figure 2Data coverage of the sites. For clarity, European and remainingstations are shown separately. Black dots on the left panel indicate thepresence of valid hourly data, and markers on the right panel indicate theperiods for which the corresponding data availability wassufficient to compute statistics .
You May Like: Is Ap Human Geography Hard
An Absolute Acidity Scale For Solvents
Comprehensive solvent acidity scale could help make acid-catalysed reactions more reliable and reproducible
Using acids from this table, buffer solutions of a well-defined composition can be prepared spanning an acidity range of over 28 pH units, which is double the pH window of water. Click here to see a larger version of this image.
A collaboration between scientists in Estonia and Germany has resulted in a comprehensive solvent acidity scale spanning 28 orders of magnitude, twice as much as the classical pH scale.
Organic chemists use the traditional pH scale to select suitable catalysts for acid-catalysed reactions carried out in water. However, acid-catalysed reactions are commonly carried-out in non-aqueous solvents of varying polarity. For these solvents, there is no general acidity scale, so trial and error is a common catalyst selection method. A comprehensive solvent acidity scale would allow for quantitative catalyst selection and give way to more convenient, reliable and reproducible reactions.
Christian Reichardt, an expert in solvent effects at the Philipps University of Marburg, Germany, agrees that this work will have wide reaching implications: The impact of this scale is not a very specific but a more general one for all kinds of acid-catalysed reactions. Having a unified Brönsted acidity scale, valid for the non-polar solvent 1,2-dichloroethane, should now allow catalyst selection using more quantitative aspects.
Examples For Acidic Organic Compounds And Comparison Of Acidity
- Alkyne compounds which have acidic hydrogen atom are more acidic than alkynes which do not have acidic hydrogen atoms. As an example, 1-butyne is more acidic than 2-butyne.
- Carboxylic acid compounds are more acidic than alcohol compounds. Example: Ethanol is less acidic than ethanoic acid
Most of the acidic organic compounds are weak acids. Dissociation constant of acids value tells us about the acidity or strength of the acid. When Ka value is high, acidic strength is high.
When we study about acidity of compounds, we have to look their reactions with following compounds and products and then observe reaction rates.
Recommended Reading: Chemistry Half Life Formula
Ario Atom Resonance Induction & Orbital
When were looking at compounds in Organic Chemistry and were looking at acidity or basicity, I really want you to remember four general principles.
These are related to the letters A, R, I, and O:
- A stands for Atom
- I is Induction
- O is Orbital
This is the general order in which I want you to think of in terms of looking at the acidity of a compound. A is more important generally than R, R is more important generally than I, and O is normally the last on the list.
Now, this becomes more clear when we look at examples. Lets look at a few examples.
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.
Don’t Miss: What Does The P Stand For In Pemdas
Pka And Dissociation Equilibrium
Acids include strong acids, which completely dissociate in water, and weak acids, which only partially dissociate. When an acid dissociates, it releases a proton to make the solution acidic, but weak acids have both a dissociated state and undissociated state that coexist according to the following dissociation equilibrium equation.
The concentration ratio of both sides is constant given fixed analytical conditions and is referred to as the acid dissociation constant . Ka is defined by the following equation.
The square brackets indicate the concentration of respective components. Based on this equation, Ka expresses how easily the acid releases a proton . In addition, the equation shows how the dissociation state of weak acids vary according to the level in the solution.Carboxylic acids , such as acetic and lactic acids, normally have a Ka constant of about 10-3 to 10-6. Consequently, expressing acidity in terms of the Ka constant alone can be inconvenient and not very intuitive.Therefore, pKa was introduced as an index to express the acidity of weak acids, where pKa is defined as follows.
For example, the Ka constant for acetic acid is 0.0000158 , but the pKa constant is 4.8, which is a simpler expression. In addition, the smaller the pKa value, the stronger the acid. For example, the pKa value of lactic acid is about 3.8, so that means lactic acid is a stronger acid than acetic acid.