Faqs On Activation Energy Formula
Q.1. What is activation energy?Ans. This activation energy can be defined as the minimum amount of external energy required to convert the reactant into the product in a chemical reaction. It is denoted as \ having S.I. unit as kilojoules per mole \\).
Q.2. What is the activation energy in a chemical equation?Ans. In chemistry, the activation energy is the minimum amount of energy that is required to activate atoms or molecules of the reactant to a condition in which they can undergo chemical reaction by effective collisions in the transition state of the chemical reaction. Lower the activation energy faster will be the conversion of intermediate to the products and vice versa. This entire phenomenon is based on the collision theory, where an effective collision of molecules is needed for a reaction to occur.
Q.3. Is activation energy positive or negative?Ans. Activation energy is always positive. It is the energy supplied to the reactant molecules for their effective collisions to form the product. Since this energy is provided in a chemical reaction, it cannot be negative for elementary reactions. It may be negative for multi-step reactions in some cases where the reaction may be too fast to control.
Q.5. What are the types of catalyst?Ans. Based on the type of changes brought by a catalyst in a chemical reaction, a catalyst is classified into a positive catalyst and a negative catalyst.
Meaning Of Activation Energy
To understand its concept, we can visualize it as the magnitude of the energy barrier i.e. the potential barrier. This barrier separates the minima of potential energy surfaces. It involves the initial and the final thermodynamic state of the system.
To define it, we have to analyze the initiation of chemical reactions. These reactions occur when molecules exchange electrons or when we bring together ions with opposite charges. To exchange electrons in the molecules, the bonds keeping the electrons tied with the molecule, have to be broken.
An external energy source can give the energy which is required to dislodge the electrons and hence can allow the chemical reaction to proceed. We express activation energy in units such as kilojoules, kilocalories or kilowatt-hours.
Once the reaction is on the way, it releases energy and then it is self-sustaining. So, we need this activation energy only at the beginning, to let the chemical reaction start.
Finding A Catalyst For Your Habits
Everyone is on the lookout for tactics and hacks that can make success easier. Chemists are no different. When it comes to dealing with chemical reactions, the one trick chemists have up their sleeves is to use what is known as a catalyst.
A catalyst is a substance that speeds up a chemical reaction. Basically, a catalyst lowers the activation energy and makes it easier for a reaction to occur. The catalyst is not consumed by the reaction itself. Its just there to make the reaction happen faster.
Heres a visual example:
When it comes to building better habits, you also have a catalyst that you can use:
Your environment.
The most powerful catalyst for building better habits is environment design . The idea is simple: the environments where we live and work influence our behaviors, so how can we structure those environments to make good habits more likely and bad habits more difficult?
Here is an example of how your environment can act as a catalyst for your habits:
Your environment can catalyze your habits in big and small ways. If you set your running shoes and workout clothes out the night before, you just lowered the activation energy required to go running the next morning. If you hire a meal service to deliver low-calorie meals to your door each week, you significantly lowered the activation energy required to lose weight. If you unplug your television and hide it in the closet, you just lowered the activation energy required to watch less television.
Also Check: Why Is Chemistry Called The Central Science
Microscopic Factor : Activation Energy
Previously, we discussed the kinetic molecular theory of gases, which showed that the average kinetic energy of the particles of a gas increases with increasing temperature. Because the speed of a particle is proportional to the square root of its kinetic energy, increasing the temperature will also increase the number of collisions between molecules per unit time. What the kinetic molecular theory of gases does not explain is why the reaction rate of most reactions approximately doubles with a 10°C temperature increase. This result is surprisingly large considering that a 10°C increase in the temperature of a gas from 300 K to 310 K increases the kinetic energy of the particles by only about 4%, leading to an increase in molecular speed of only about 2% and a correspondingly small increase in the number of bimolecular collisions per unit time.
The collision model of chemical kinetics explains this behavior by introducing the concept of activation energy ). We will define this concept using the reaction of \ with ozone, which plays an important role in the depletion of ozone in the ozone layer:
Increasing the temperature from 200 K to 350 K causes the rate constant for this particular reaction to increase by a factor of more than 10, whereas the increase in the frequency of bimolecular collisions over this temperature range is only 30%. Thus something other than an increase in the collision rate must be affecting the reaction rate.
\ \nonumber \]
Activation Energy And Reaction Rate
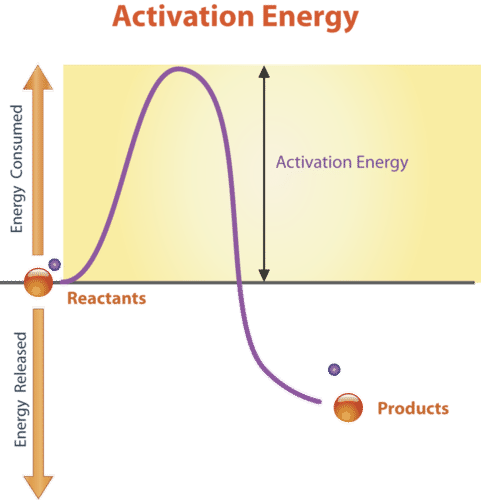
The activation energy of a chemical reaction is closely connected to its reaction rate. The higher the activation energy, the slower the reaction will be. For example, the rusting of iron is a long process because the activation energy for this reaction is high .
According to the kinetic theory of matter, molecules have a wide range of kinetic energy. Only a few molecules have enough energy to overcome the activation energy barrier. The higher the activation energy, the smaller the fraction of molecules that can overcome this barrier. Since fewer molecules have reached this level, the reaction will progress slowly. For this reason, many reactions do not proceed at all at room temperature.
Let us look at a few real-life examples to understand reaction rates better. The combustion of propane does not occur unless ignited by fire. Wood does not burn unless lighted by a match. These combustion reactions do not occur spontaneously. In both these cases, the temperature is raised above the room temperature. Thus, having high activation energies for these reactions is favorable. Generally, high temperature and low activation energy result in a large reaction rate.
Catalyst and Enzymes
Enzymes are biological catalysts made out of proteins found in the human body. They work by lowering the activation energy and speeding up chemical reactions in the body. They bind the reactant molecules and orient them in a specific manner to initiate the reactions.
You May Like: Springboard Algebra 1 Unit 1 Answers
What Happens When There Isnt Enough Activation Energy
Although it is possible for chemical reactions to occur naturally, it is a rare event. This is because the majority of molecules in nature have low energy levels, resulting in stable bonds. These low energy bonds make it difficult for the molecule to be pushed into a transition state unless they are given enough activation energy.
Formula Of Activation Energy
The activation energy is equal to the difference between the threshold energy needed for the reaction to occur and the average kinetic energy of all the reacting molecules in reactant species. i.e.,\
This means if the activation energy for a reaction is low, the fractions of effective collisions are large, having high threshold energy, and the rate of a reaction is high. If the activation energy is high, the number of effective collisions is small, and the rate of the reaction is slow.
Thus, to conclude:
Based on Arrhenius concept: Activation Energy is dependent on various factors out of them, the temperature is the most important one. The Arrhenius equation deals with the quantitative basis of the relationship between the activation energy and the rate of a chemical reaction proceeds. The formula of Activation energy is given below:\
In the above equation, the notations are:
The Arrhenius equation is regarded as the best experimentally determined parameter that indicates the sensitivity of the reaction rate to temperature and activation energy.
Basically, activation energy is the potential barrier or energy barrier that the reactant molecules need to achieve to proceed in a chemical reaction.
Also Check: Who Wrote The Early Textbook Principles Of Psychology
Activation Energy: Why Getting Started Is The Hardest Part
The beginning of any complex or challenging endeavor is always the hardest part. Not all of us wake up and jump out of bed ready for the day. Some of us, like me, need a little extra energy to transition out of sleep and into the day. Once Ive had a cup of coffee, my energy level jumps and Im good for the rest of the day. Chemical reactions work in much the same way. They need their coffee, too. We call this activation energy.
Understanding how this works can be a useful perspective as part of our latticework of mental models.
Whether you use chemistry in your everyday work or have tried your best not to think about it since school, the ideas behind activation energy are simple and useful outside of chemistry. Understanding the principle can, for example, help you get kids to eat their vegetables, motivate yourself and others, and overcome inertia.
The Activation Energy Of Building Better Habits
Similar to how every chemical reaction has an activation energy, we can think of every habit or behavior as having an activation energy as well.
This is just a metaphor of course, but no matter what habit you are trying to build there is a certain amount of effort required to start the habit. In chemistry, the more difficult it is for a chemical reaction to occur, the bigger the activation energy. For habits, its the same story. The more difficult or complex a behavior, the higher the activation energy required to start it.
For example, sticking to the habit of doing 1 pushup per day requires very little energy to get started. Meanwhile, doing 100 pushups per day is a habit with a much higher activation energy. It’s going to take more motivation, energy, and grit to start complex habits day after day.
Also Check: What Is Direct Proportion In Physics
Activation Energy And Gibbs Energy
The Eyring equation is another relation describing the rate of reaction. However, the equation uses Gibbs energy of the transition state rather than activation energy. The Gibbs energy of the transition state accounts for the enthalpy and entropy of a reaction. While activation energy and Gibbs energy are related, they arent interchangeable in chemical equations.
Activation Energy And The Activated Complex
If you take a match and just hold it or wave it around in the air, the match will not light. You have to strike the match against the side of the box. All chemical reactions need something that makes them start going.
Chemical reactions will not take place until the system has some minimum amount of energy added to it. This energy is called the activation energy.
- Activation energy
-
Activation energy is the minimum amount of energy that is needed to start a chemical reaction.
It is important to realise that even though exothermic reactions release energy they still need a small amount of energy to start the reaction.
Recall from earlier that we drew graphs for the energy changes in exothermic and endothermic reactions. We can now add some information to these graphs. This will also explain why we draw these graphs with a curve rather than using a straight line from the reactants energy to the products energy.
We will start by looking at exothermic reactions. We will use:
as an example of an exothermic reaction.
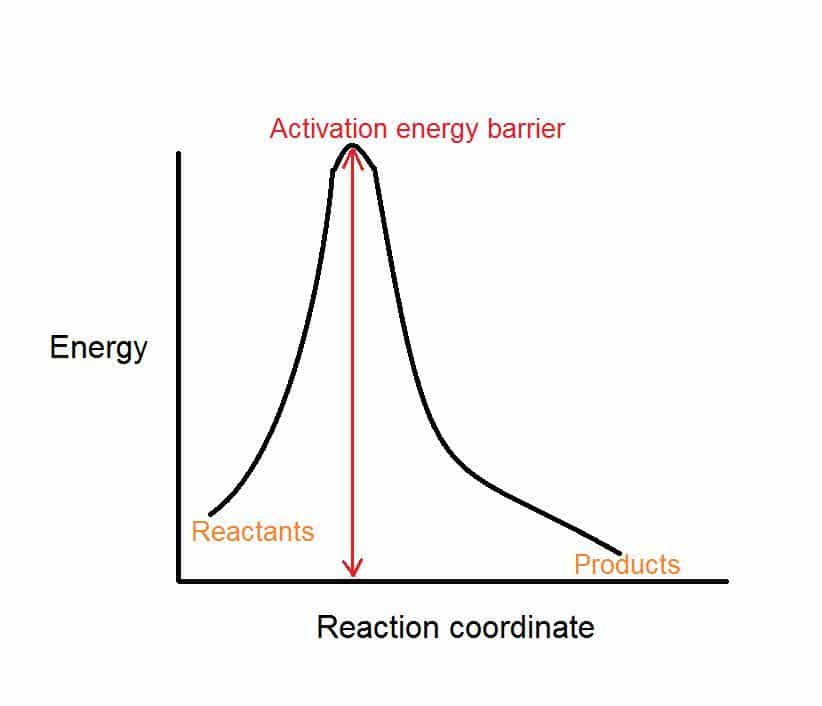
The activation energy is the difference between the energy of the reactants and the maximum energy .
The reaction between \\) and \\) needs energy in order to proceed, and this is the activation energy. To form the product the bond between \ and \ in \ must break. The bond between \ and \ in \ must also break. A new bond between \ and \ must also form to make \. The reactant bonds break at the same time that the product bonds form.
We can show this as:
Don’t Miss: What Is Microevolution In Biology
Solved Example For You
Question- Find out the activation energy, considering that k = \, T = 400 K. Also, the pre-exponential factor here is \.
Answer- k = \RT\) = \
R happens to be a gas constant whose value is 8.314 J/ molK, therefore
\8.314j/molK400K\) = Ea
Ea = 21.91 kJ/mol
Hence, the activation energy is 21.91 kJ/mol.
What Is Activation Energy Definition And Examples
In chemistry and physics, activation energy is the minimum amount of energy needed to start a chemical reaction. Reactants often get activation energy from heat, but sometimes energy comes from light or energy released by other chemical reactions. For spontaneous reactions, the ambient temperature supplies enough energy to achieve the activation energy.
Swedish scientist Svante Arrhenius proposed the concept of activation energy in 1889. Activation energy is indicated by the symbol Ea and has units of joules , kilojoules per mole , or kilocalories per mole .
Also Check: What Are Fundamental Quantities In Physics
Microscopic Factor : Collisional Frequency
Central to collision model is that a chemical reaction can occur only when the reactant molecules, atoms, or ions collide. Hence, the observed rate is influence by the frequency of collisions between the reactants. The collisional frequency is the average rate in which two reactants collide for a given system and is used to express the average number of collisions per unit of time in a defined system. While deriving the collisional frequency ) between two species in a gas is straightforward, it is beyond the scope of this text and the equation for collisional frequency of \ and \ is the following:
with
- \ and \ are the numbers of \ and \ molecules in the system, respectively
- \ and \ are the radii of molecule \ and \, respectively
- \ is the Boltzmann constant \ =1.380 x 10-23 Joules Kelvin
- \ is the temperature in Kelvin
- \ is calculated via \
The specifics of Equation \ref are not important for this conversation, but it is important to identify that \ increases with increasing density and \), with increasing reactant size and \), with increasing velocities /09%3A_Gases/9.5%3A_The_Kinetic-Molecular_Theory” rel=”nofollow”> Kinetic Molecular Theory), and with increasing temperature .
A Video Discussing Collision Theory of Kinetics: Collusion Theory of Kinetics
Macroscopic Behavior: The Arrhenius Equation
The collision model explains why most collisions between molecules do not result in a chemical reaction. For example, nitrogen and oxygen molecules in a single liter of air at room temperature and 1 atm of pressure collide about 1030 times per second. If every collision produced two molecules of \, the atmosphere would have been converted to \ and then \ a long time ago. Instead, in most collisions, the molecules simply bounce off one another without reacting, much as marbles bounce off each other when they collide.
For an \ elementary reaction, all three microscopic factors discussed above that affect the reaction rate can be summarized in a single relationship:
where
\ \label \]
Arrhenius used these relationships to arrive at an equation that relates the magnitude of the rate constant for a reaction to the temperature, the activation energy, and the constant, \, called the frequency factor:
The frequency factor is used to convert concentrations to collisions per second . Because the frequency of collisions depends on the temperature, \ is actually not constant . Instead, \ increases slightly with temperature as the increased kinetic energy of molecules at higher temperatures causes them to move slightly faster and thus undergo more collisions per unit time.
If we know the reaction rate at various temperatures, we can use the Arrhenius equation to calculate the activation energy. Taking the natural logarithm of both sides of Equation \,
\ & =\ln A+\left \label \end \]
You May Like: What Is Sample Space In Math Terms
Activation Energy: The Chemistry Of Getting Started
Don’t have time to read this now? Use Matter to read it later, listen on the go, or even send to Kindle.
In chemistry, activation energy is the energy that must be provided to result in a chemical reaction. The more energy is needed, the harder it is to start the chemical reaction. In work and life as well, we sometimes need to get over the initial hump of getting started.
For instance, it can be hard to get out of bed, or to start studying for an exambut once you get started, you find it quite easy to keep going with a task. The activation energy metaphor can be helpful to understand why the most challenging part is often to get started. How much energy do you need? Which specific catalysts would be helpful?
You may not feel outstandingly robust, but if you are an average-sized adult you will contain within your modest frame no less than 7 x 1018 joules of potential energyenough to explode with the force of thirty very large hydrogen bombs, assuming you knew how to liberate it and really wished to make a point. Bill Bryson, A Short History of Nearly Everything.
How To Use A Graph To Find Activation Energy
Another way to calculate the activation energy of a reaction is to graph ln k versus 1/T . The plot will form a straight line expressed by the equation:
m = – Ea/R
where m is the slope of the line, Ea is the activation energy, and R is the ideal gas constant of 8.314 J/mol-K. If you took temperature measurements in Celsius or Fahrenheit, remember to convert them to Kelvin before calculating 1/T and plotting the graph.
If you were to make a plot of the energy of the reaction versus the reaction coordinate, the difference between the energy of the reactants and the products would be H, while the excess energy would be the activation energy.
Keep in mind, while most reaction rates increase with temperature, there are some cases where the rate of reaction decreases with temperature. These reactions have negative activation energy. So, while you should expect activation energy to be a positive number, be aware that it’s possible for it to be negative as well.
Also Check: What To Study For Tsi Math Test