What Is The Most Dangerous Pollution
Undoubtedly air pollution is the most devastating pollution type causing worldwide mortality. An estimated 7 million deaths every year are linked to ambient air pollution, mainly from heart disease, stroke, chronic obstructive pulmonary disease , lung cancer, and respiratory infections, including pneumonia.
Regulation And Emission Control Technologies
Selective catalytic reduction and selective non-catalytic reduction reduce post combustion NOx by reacting the exhaust with urea or ammonia to produce nitrogen and water. SCR is now being used in ships, diesel trucks and in some diesel cars. The use of exhaust gas recirculation and catalytic converters in motor vehicle engines have significantly reduced vehicular emissions. NOx was the main focus of the Volkswagen emissions violations.
Other technologies such as flameless oxidation and staged combustion significantly reduce thermal NOx in industrial processes. Bowin low NOx technology is a hybrid of staged-premixed-radiant combustion technology with a major surface combustion preceded by a minor radiant combustion. In the Bowin burner, air and fuel gas are premixed at a ratio greater than or equal to the stoichiometric combustion requirement.Water Injection technology, whereby water is introduced into the combustion chamber, is also becoming an important means of NOx reduction through increased efficiency in the overall combustion process. Alternatively, the water is emulsified into the fuel oil before the injection and combustion. This emulsification can either be made in-line just before the injection or as a drop-in fuel with chemical additives for long term emulsion stability .
Oxidation Of Nh2oh By Fe
Iron not only mediates NO and N2O production from NO2. As Fe, it also oxidizes NH2OH to N2O. This process can be used for the analytical determination of trace amounts of NH2OH . The general equation for the reaction is
In this reaction, N2O formation strongly depends on the pH value. In experiments with distilled water and natural seawater, Butler and Gordon found that at pH 3, N2O recovery was 80%, while at a pH value of 9.5, N2O production was negligibly low. The authors hypothesized that at high pH values, HNO, reacts with O2 to produce NO2 and H2O. However, it is also known that HNO can react with NH2OH to N2 . Chemical production of N2O via NH2OH oxidation by Fe is a likely process during nitrification, because Fe compounds are ubiquitous in natural waters and wastewater treatment systems.
Read Also: What Are The Major Specialties In The Field Of Psychology
A Need For An Integrated Approach To No And N2o Turnover In Complex Microbial Communities
NO and N2O can be produced by many different biological and chemical reactions. Considerable progress has been made to allocate NO and N2O production to certain biological pathways, but commonly some uncertainty remains, because many processes share the same reaction sequence for N2O production via NO and NO2. We delineated basically three-independent approaches to allocate pathways . Parallel use of these approaches will increase confidence in the interpretation. The possibility for various chemical reaction that produce and consume NO and N2O additionally complicate the picture. Chemical reactions can be important in engineered systems that employ waters with concentrated N-contents and in natural systems, where low pH values coincide with high ammonia inputs. However, in most natural systems and in municipal wastewater treatment, chemical reactions will probably not be the main contributors of NO and N2O emissions. Nevertheless, the possibility of chemical NO and N2O production has to be considered when interpreting measurements results. Experiments with inactivated biomass could help to give a first estimation of the chemical production rates. However, care has to be taken since the chemical conditions that facilitate chemical NO and N2O production such as pH and trace metal availability are in turn shaped by microbial activity.
What Is Zinc Oxide
Zinc Oxide is an inorganic compound which is also known as Calamine or Zinc White. It is naturally found as a mineral zincite. It is mostly produced synthetically. It is a chemical compound with mild astringent and antiseptic action which works as a topical protectant. It has wide applications in bandages, ointments, pastes, and dental cement.
Don’t Miss: Ccl4lewis Structure
Nitric Oxide And Nitrous Oxide Turnover In Natural And Engineered Microbial Communities: Biological Pathways Chemical Reactions And Novel Technologies
- 1Department of Environmental Microbiology, Eawag – Swiss Federal Institute of Aquatic Science and Technology, Dübendorf, Switzerland
- 2Department of Environmental Systems Sciences, Eidgenössische Technische Hochschule, Zurich, Switzerland
- 3Department of Process Engineering, Eawag – Swiss Federal Institute of Aquatic Science and Technology, Dübendorf, Switzerland
- 4Department of Civil, Environmental and Geomatic Engineering, Eidgenössische Technische Hochschule, Zurich, Switzerland
What Is Not An Oxide
In order to be an oxide, the oxidation state of oxygen must be -2 and the oxygen must act as an anion. The following ions and compounds are not technically oxides because they don’t meet these criteria:
- Oxygen difluoride : Fluorine is more electronegative than oxygen, so it acts as the cation rather than the anion in this compound.
- Dioxygenyl and its compounds: Here, the oxygen atom is in the +1 oxidation state.
You May Like: Geometry Segment Addition Postulate Worksheet
Quantifying The Genetic Potential For N2o Consumption
An appealing focus for application of molecular tools in environmental samples is direct quantification via the quantitative polymerase chain reaction of relevant functional genes . Such an approach most commonly targets DNA, not RNA, and is thus a measure of genetic potential in the environment and not the activity.
Table 4. Reported primers and literature references relevant for NO and N2O turnover during N-cycling.
In contrast, uhel et al. detail a significant but, puzzlingly, positive correlation in grassland soil between nosZ/ ratios and N2O/, but caution that the relative importance of denitrifier community composition and enzyme regulation relative to proportion of nosZ deficient community members remains uncertain. In line with this result, Braker and Conrad found similar ratios of nosZ/ via Most Probable Number PCR in three soils with profoundly different N2O/ ratios, and concluded that the hypothesis that a higher abundance of denitrifiers lacking nosZ is linked to increased N2O emissions may be an oversimplification.
The genetic potential for N2O production via nitrifier denitrification in AOB could theoretically be measured via qPCR of the nirK and norB genes. Design of such analyses is hampered due to the fact that AOB nirK and norB genes are not phylogenetically distinct from that of heterotrophic denitrifying organisms . In addition, NorB is not the only NO reductase in AOB .
Is Nitrogen Dioxide Heavier Than Air
At high concentrations, nitric oxide is quickly oxidized into the air to produce nitrogen dioxide. Expositions. Nitrogen dioxide is heavier than air, so exposure can result in asphyxiation in poorly ventilated, sealed, or low-lying areas. The gasses at room temperature are both nitrogen dioxide and nitric oxide.
You May Like: Why Are There Different Branches Of Chemistry
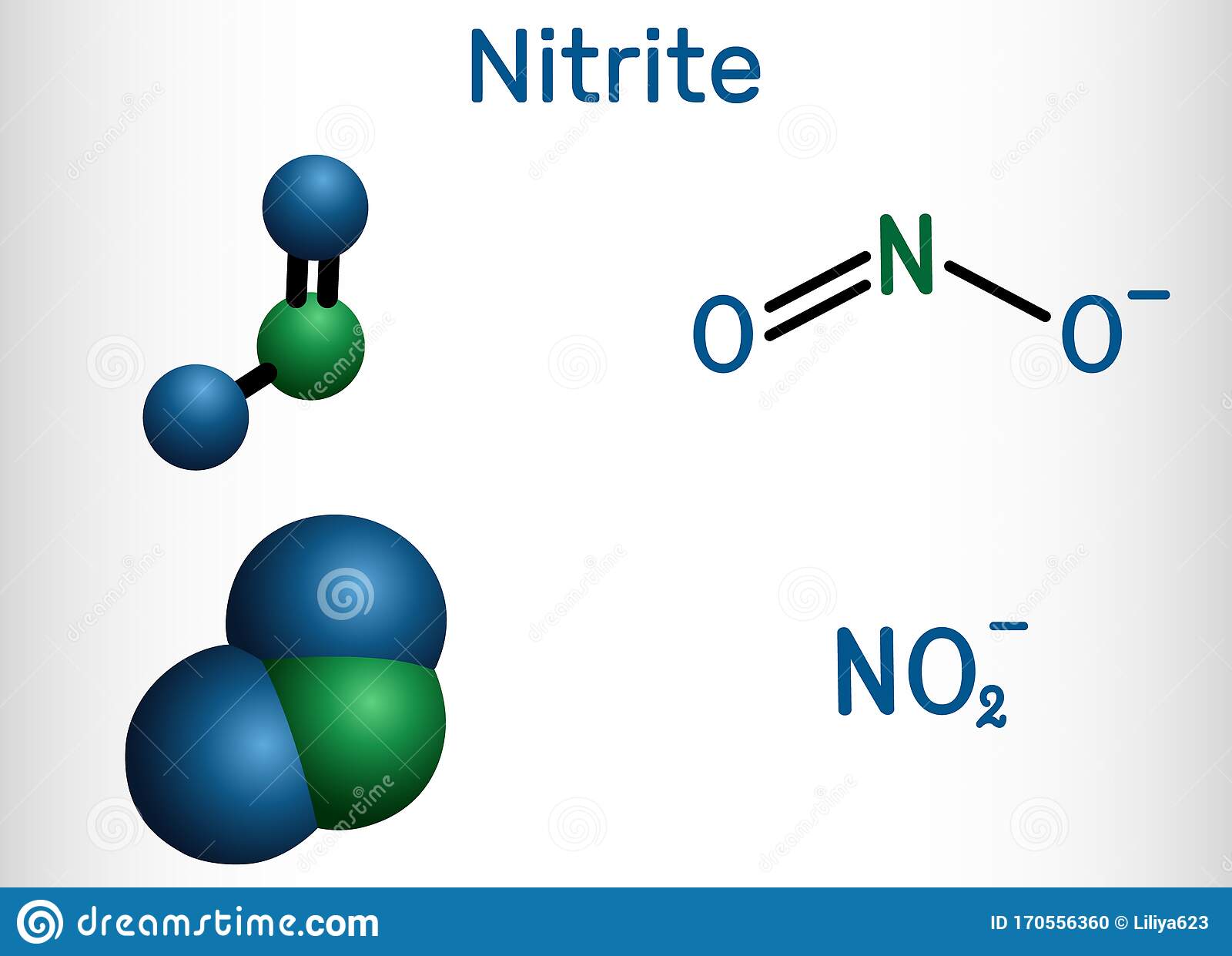
Meet The Nitrogen Oxide Family: Nox No And No2
Meet nitrogen, commonly referred to simply with the chemical symbol N. When two nitrogen atoms bond together, they form nitrogen gas . Nitrogen gas is odourless, colourless and tasteless. It is non-flammable and will not support combustion. Fun fact: nitrogen makes up 78% of the Earths atmosphere. You probably remember learning about the nitrogen cycle in school and how it is crucial to all life on earth.
Meet nitrogens friend oxygen , a highly reactive non-metal, oxidizing agent that readily forms oxides with most elements and other compounds. At normal pressure and temperature, molecules of oxygen bind to form dioxygen which is present in the atmosphere.
Oxygen is the third most abundant element in the universe, and of course is crucial to our continued existence. Interesting fact: the most common auroral colour in the northern lights is produced by oxygen molecules located about 60 miles above the earth. How cool is that!
Application Of No Microelectrodes
The novel NO microelectrode has been applied to study NO formation in permeable marine and river sediments. The results showed that in steady-state NO is produced in oxic/micro-oxic sediment strata reaching concentrations of 0.13 mol L1 in river and 0.5 mol L1 in marine sediments. In both sediments, NO produced in the oxic zone was consumed in the anoxic zone. It was hypothesized that NO was produced by AOB in the oxic zone. Labeling experiments with a 15N-labeled NO donor in the river sediment suggested that denitrification actively consumes exogenously produced NO.
Read Also: Eoc Fsa Practice Test Algebra 1 No Calculator Portion
Anaerobic Methane And Ammonia Oxidizing Bacteria
Bacteria that mediate the oxygenic nitrite-dependent oxidation of methane and anaerobic ammonia oxidation have been shown to use NO as an intracellular intermediate produced by NO2 reduction via NirS while they consume exogenous NO without concurrent N2O formation . Rather, N-AOM dismutates NO to form N2 and O2, while anammox couples the reduction of NO to a condensation with NH3 to produce hydrazine . Both have the genetic potential to reduce NO to N2O anammox bacteria encode for flavorubredoxin and N-AOM encodes for qNor . However, physiological data for both indicates that they withstand rather high NO levels without activating anaerobic NO detoxification mechanisms.
A Role For Variation In Regulatory Response
Differences in transcriptional and translational regulation as well as enzyme activity have also been highlighted as potentially critical modulators of microbial NO or N2O production . Such differences likely contribute to observed associations between community structure and greenhouse gas production discussed above. Strong regulation at the transcriptional, translational, and enzyme level is likely occurring in both nitrifier and denitrifier communities, and such regulation complicates attempts to directly relate abundance or diversity of functional guilds to process rates . Similarly, transient near-instantaneous NO and N2O accumulation in active nitrifying and denitrifying biofilms in response to O2 or NO2 perturbations, as measured with high temporal resolution via microelectrodes, strongly suggests that dynamics are controlled in some cases at the enzyme level . Indeed, culture-based assays targeting denitrifier isolates from two soils demonstrated substantial diversity in sensitivity of Nos enzymes to O2 and provided a physiological underpinning for a previously observed link between denitrifier community composition and rate of N2O production .
Read Also: Imagine Math Username And Password
Physical Properties Of Zinc Oxide
Zinc white crystallizes mainly in two forms viz cubic zinc blende and hexagonal wurtzite. The most common and stable structure at ambient conditions is wurtzite. Zincblende can be stabilized by growing zinc oxide on substrates which has a cubic lattice structure. The oxide and zinc centres are tetrahedral.
Where Do High No2 Concentrations Occur
Monitors show the highest concentrations of outdoor NO2 in large urban regions such as the Northeast corridor, Chicago and Los Angeles.6 Levels are higher on or near heavily traveled roadways.
NO2 can be a problem indoors, as well. Kerosene or gas space heaters and gas stoves also produce substantial amounts of nitrogen dioxide. If those heaters or stoves are not vented fully to the outside, levels of NO2 can build up indoors.
- References
U.S. Environmental Protection Agency. Integrated Science Assessment for Oxides of Nitrogen — Health Criteria. EPA/600/R-15/068. January 2016. Available at: https://cfpub.epa.gov/ncea/isa/recordisplay.cfm?deid=310879
U.S. EPA, 2016.
Eckel SP, Cockburn M, Shu Y-H, et al. F. Air pollution affects lung cancer survival. Thorax. 2016: 71: 891-898.
U.S. EPA, 2016.
U.S. EPA. Air Emissions Sources: Nitrogen Oxides, National Summary of Nitrogen Oxides Emissions, 2011. Available at
U.S. EPA. Risk and Exposure Assessment to Support the Review of the NO2 Primary National Ambient Air Quality Standard. EPA-452/R-08-008a, November 2008. Available at:
Don’t Miss: Draw A Lewis Structure (including All Lone Pair Electrons) For The Species Ccl4:
Nitrogen Oxides As Pollutants
Nitrogen oxides are at least partially responsible for several types of air pollution. Nitrogen dioxide lends its color to the reddish-brown haze we call smog. Photodissociation of nitrogen dioxide by sunlight produces nitric oxide and ozone in the troposphere, which is another component of smog. A series of chemical reactions transform Volatile Organic Compounds into substances that combine with nitrogen dioxide to produce PAN , yet another element in smog. Nitrogen dioxide in the air also reacts with water vapor to form nitric acid, one of the types of acid in acid rain.
Nitrogen dioxide concentration in unpolluted air is around 10 parts per billion . In smog, the concentration rises twenty-fold to about 200 ppb.
What Gets Stored In A Cookie
This site stores nothing other than an automatically generated session ID in the cookie no other information is captured.
In general, only the information that you provide, or the choices you make while visiting a web site, can be stored in a cookie. For example, the site cannot determine your email name unless you choose to type it. Allowing a website to create a cookie does not give that or any other site access to the rest of your computer, and only the site that created the cookie can read it.
Recommended Reading: What Is Geology In Geography
Human Sources Of Nitrogen Oxides
At normal temperatures the oxygen and nitrogen gases do not react together. In the presence of very high temperatures nitrogen and oxygen do react together to form nitric oxide. These conditions are found in the combustion of coal and oil at electric power plants, and also during the combustion of gasoline in automobiles. Both of these sources contribute about equally to the formation of nitrogen oxides.
In areas of high automobile traffic, such as in large cities, the amount of nitrogen oxides emitted into the atmosphere can be quite significant. In the Los Angeles area, the main source of acid rain is from automobiles. In certain national parks such as Yosemite and Sequoia, automobile traffic is banned to limit the amount of air pollution damage to the trees and plants. This also has the effect of reducing the visual smog in the air.
What Are The Sources Of Nitrogen Dioxide Emissions
Cars, trucks, and buses are the largest sources of emissions, followed by power plants, diesel-powered heavy construction equipment and other movable engines, and industrial boilers. Man-made sources in the U.S. emitted 14 million metric tons of nitrogen oxides, mainly from burning fuels, in 2011.5 Emissions of nitrogen dioxide will decline as cleanup of many of these sources continue in future years.
Recommended Reading: Eoc Fsa Warm Ups Algebra 1 Answers
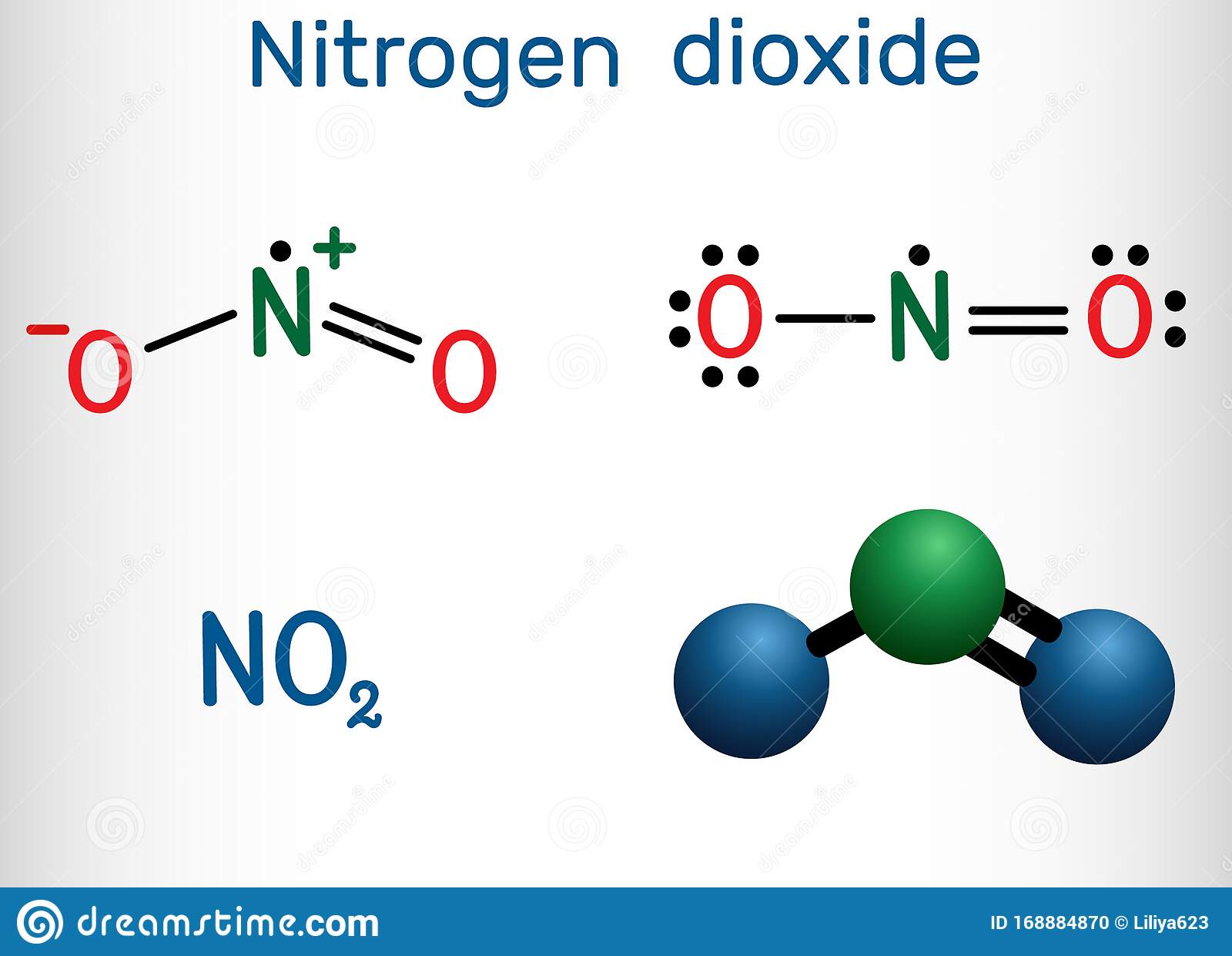
Chemical Properties Of Nitrogen Dioxide No2
1. Thermal properties Exists in equilibrium dinitrogen tetroxide gas:
2 NO2 N2O4
2. As an oxidizer Due to the weakness of the NO bond, NO2 is a strong oxidizer.
3. Hydrolysis reaction Hydrolysis reaction produces nitrous acid and nitric acid.
2 NO2 + H2O HNO2 + HNO3
5. It is a negligibly slow reaction at low concentrations of nitrogen dioxide.
6. Formation of nitrites corresponding nitrites are formed by alkyl and metal iodides.
2 CH3I + 2 NO2 2 CH3NO2 + I2
TiI4 + 4 NO2 Ti4 + 2 I2
Difference Between N2o4 And No2
November 22, 2020 Posted by Madhu
The key difference between N2O4 and NO2 is that N2O4 is diamagnetic, whereas NO2 is paramagnetic.
N2O4 is dinitrogen tetroxide while NO2 is nitrogen dioxide. Although the chemical formula N2O4 can be obtained by doubling the stoichiometric values of the chemical formula NO2, these two are different chemical compounds with different chemical and physical properties.
Don’t Miss: Elastic Force Physics
Chemical Properties Of Zinc Oxide
Calamine is a white solid which is insoluble in water and has no smell. Crude zinc oxide appears yellow-grey in colour which exists in a granular solid form with no odour. In its natural form, it is obtained as a mineral zincite which consists of manganese and some other impurities which makes it appear yellow-red colour. In its crystalline form, it is thermochromic which on heating in the presence of air changes its colour from white to yellow and on cooling it turns white in colour. It is an amphoteric oxide which is insoluble in water. It dissolves in acids and alkalis.
How Does The Presence Of Nitrogen Oxides Impact Me Are There Any Adverse Effects On My Health And Should I Be Concerned
Nitrogen oxides react to form smog and acid rain. NOx reacts with ammonia, moisture and other compounds to form nitric acid vapour and related particles. The impacts of NOx on human health include damage to the lung tissue, breathing and respiratory problems.
Nitric oxide is not considered to be hazardous to health at typical ambient conditions. However, excess nitric oxide and its products may cause respiratory ailments, hematologic side effects, metabolic disorders, low blood pressure, nausea, vomiting and diarrhoea.
Nitrogen dioxide at high concentrations causes inflammation of the airways. Breathing in high levels of NO2 can increase the likelihood of respiratory problems: wheezing, coughing, colds, flu and bronchitis. People with asthma are prone to have more intense attacks. Prolonged exposure to high levels of NO2 can cause irreversible damages to the respiratory system.
Read Also: Do You Capitalize Bachelor’s Degree In Psychology
Setting Your Browser To Accept Cookies
There are many reasons why a cookie could not be set correctly. Below are the most common reasons:
- You have cookies disabled in your browser. You need to reset your browser to accept cookies or to ask you if you want to accept cookies.
- Your browser asks you whether you want to accept cookies and you declined. To accept cookies from this site, use the Back button and accept the cookie.
- Your browser does not support cookies. Try a different browser if you suspect this.
- The date on your computer is in the past. If your computer’s clock shows a date before 1 Jan 1970, the browser will automatically forget the cookie. To fix this, set the correct time and date on your computer.
- You have installed an application that monitors or blocks cookies from being set. You must disable the application while logging in or check with your system administrator.